Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grape Seed Proanthocianidin-Rich Extract
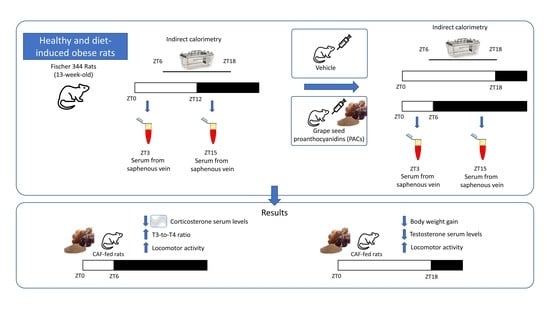
2.2. Animal Experiment Procedure
2.3. Indirect Calorimetry
2.4. Hormone Analysis
2.5. Statistical Analysis
3. Results
3.1. Abrupt Photoperiod Transfer Caused Changes in Body Weight Gain and Food Intake Patterns in CAF-Fed Rats: Effects of Grape Seed Proanthocyanidins
3.2. Grape Seed Proanthocyanidins Attenuated the Impact of Photoperiod Change on Energetic Expenditure
3.3. Cafeteria Diet Decreases the Activity of Animals in Standard Conditions and Grape Seed Proanthocyanidins Attenuated Photoperiod Changes in the Activity of Rats
3.4. GSPE Modulates Rhythm Alterations Caused by Abrupt Photoperiod Change in Serum Hormones
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Young, M.; McGinnis, G. Circadian regulation of metabolic homeostasis: Causes and consequences. Nat. Sci. Sleep 2016, 8, 163–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Levi, F.; Schibler, U. Circadian Rhythms: Mechanisms and Therapeutic Implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef]
- Challet, E. Circadian clocks, food intake, and metabolism. Prog. Mol. Biol. Transl. Sci. 2013, 119, 105–135. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [Green Version]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, L.; Jochum, S.B.; Shaikh, M.; Wilber, S.; Zhang, L.; Hayden, D.M.; Forsyth, C.B.; Voigt, R.M.; Bishehsari, F.; Keshavarzian, A.; et al. Circadian misalignment by environmental light/dark shifting causes circadian disruption in colon. PLoS ONE 2021, 16, e0251604. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of altered photoperiod on circadian clock and lipid metabolism in rats. Chrono-Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef]
- Tsai, L.-L.; Tsai, Y.-C.; Hwang, K.; Huang, Y.-W.; Tzeng, J.-E. Repeated light-dark shifts speed up body weight gain in male F344 rats. Am. J. Physiol. Metab. 2005, 289, E212–E217. [Google Scholar] [CrossRef]
- López-Espinoza, A.; Gallardo, A.C.E.; Vázquez-Cisneros, L.C.; Zepeda-Salvador, A.P.; Santillano-Herrera, D. Light-dark cycle inversion effect on food intake and body weight in rats. Nutr. Hosp. 2021, 38, 495–501. [Google Scholar] [CrossRef]
- Palacios-Jordan, H.; Martín-González, M.Z.; Suárez, M.; Aragonès, G.; Mugureza, B.; Rodríguez, M.A.; Bladé, C. The disruption of liver metabolic circadian rhythms by a cafeteria diet is sex-dependent in Fischer 344 rats. Nutrients 2020, 12, 1085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panchal, S.K.; Brown, L. Rodent Models for Metabolic Syndrome Research. J. Biomed. Biotechnol. 2010, 2011, 351982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendoza, J.; Pévet, P.; Challet, E. High-fat feeding alters the clock synchronization to light. J. Physiol. 2008, 586, 5901–5910. [Google Scholar] [CrossRef] [PubMed]
- Barnea, M.; Madar, Z.; Froy, O. High-fat diet followed by fasting disrupts circadian expression of adiponectin signaling pathway in muscle and adipose tissue. Obesity 2010, 18, 230–238. [Google Scholar] [CrossRef]
- Barnea, M.; Madar, Z.; Froy, O. High-Fat Diet Delays and Fasting Advances the Circadian Expression of Adiponectin Signaling Components in Mouse Liver. Endocrinology 2009, 150, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Parsons, M.J.; Moffitt, T.E.; Gregory, A.M.; Goldman-Mellor, S.; Nolan, P.M.; Poulton, R.; Caspi, A. Social jetlag, obesity and metabolic disorder: Investigation in a cohort study. Int. J. Obes. 2015, 39, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock genes in the metabolism of non-alcoholic fatty liver disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [Green Version]
- Kolbe, I.; Leinweber, B.; Brandenburger, M.; Oster, H. Circadian clock network desynchrony promotes weight gain and alters glucose homeostasis in mice. Mol. Metab. 2019, 30, 140–151. [Google Scholar] [CrossRef]
- Panda, S. The arrival of circadian medicine. Nat. Rev. Endocrinol. 2019, 15, 67–69. [Google Scholar] [CrossRef]
- McLean, S.L.; Yun, H.; Tedder, A.; Helfer, G. The effect of photoperiod and high fat diet on the cognitive response in photoperiod-sensitive F344 rats. Physiol. Behav. 2021, 239, 113496. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and much more! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Aragonès, G.; Arola-Arnal, A.; Muguerza, B.; Bravo, F.I.; Salvadó, M.J.; Arola, L.; Suárez, M. Proanthocyanidins in health and disease. BioFactors 2016, 42, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bladé, C.; Arola-Arnal, A.; Crescenti, A.; Suárez, M.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Arola, L. Proanthocyanidins and Epigenetics. Handb. Nutr. Diet. Epigenet. 2019, 3, 1933–1956. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, J.; Arola, L.; Bladé, C. Chronic consumption of dietary proanthocyanidins modulates peripheral clocks in healthy and obese rats. J. Nutr. Biochem. 2015, 26, 112–119. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, J.; Arola, L.; Bladé, C. Dietary proanthocyanidins modulate melatonin levels in plasma and the expression pattern of clock genes in the hypothalamus of rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvado, J.; Bladé, C.; Arola, L. Dietary proanthocyanidins modulate BMAL1 acetylation, Nampt expression and NAD levels in rat liver. Sci. Rep. 2015, 5, 10954. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, R.M.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Feillet-Coudray, C.; Casas, F.; Colom-Pellicer, M.; Aragonès, G.; Avila-Román, J.; Muguerza, B.; Mulero, M.; et al. Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats. Nutrients 2022, 14, 774. [Google Scholar] [CrossRef]
- Ávila-Román, J.; Soliz-Rueda, J.R.; Bravo, F.I.; Aragonès, G.; Suárez, M.; Arola-Arnal, A.; Mulero, M.; Salvadó, M.-J.; Arola, L.; Torres-Fuentes, C.; et al. Phenolic compounds and biological rhythms: Who takes the lead? Trends Food Sci. Technol. 2021, 113, 77–85. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Fernández-Vallinas, S.; Pons, Z.; Arola, L.; Aleixandre, A.; Muguerza, B. Involvement of nitric oxide and prostacyclin in the antihypertensive effect of low-molecular-weight procyanidin rich grape seed extract in male spontaneously hypertensive rats. J. Funct. Foods 2014, 6, 419–427. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Acute administration of single oral dose of grape seed polyphenols restores blood pressure in a rat model of metabolic syndrome: Role of nitric oxide and prostacyclin. Eur. J. Nutr. 2015, 55, 749–758. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraro, F.; Stuart, C.A.; Hartl, W.H.; Rosenblatt, J.; Wolfe, R.R. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am. J. Physiol. Metab. 1990, 259, E470–E476. [Google Scholar] [CrossRef] [PubMed]
- Atwater, W.O. Coefficients of digestibility and availability of the nutrients of food. Proc. Am. Physiol. Soc. 1909, 30, 14–19. [Google Scholar]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- Gadacha, W.; Ben-Attia, M.; Bonnefont-Rousselot, D.; Aouani, E.; Ghanem-Boughanmi, N.; Touitou, Y. Resveratrol opposite effects on rat tissue lipoperoxidation: Pro-oxidant during day-time and antioxidant at night. Redox Rep. 2009, 14, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Arola-Arnal, A.; Cruz-Carrión, Á.; Torres-Fuentes, C.; Ávila-Román, J.; Aragonès, G.; Mulero, M.; Bravo, F.I.; Muguerza, B.; Arola, L.; Suárez, M. Chrononutrition and Polyphenols: Roles and Diseases. Nutrients 2019, 11, 2602. [Google Scholar] [CrossRef] [Green Version]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Lalanza, J.F.; Snoeren, E.M. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci. Biobehav. Rev. 2021, 122, 92–119. [Google Scholar] [CrossRef]
- Escobar, C.; Espitia-Bautista, E.; Guzmán-Ruiz, M.A.; Vargas, N.N.G.; Hernández-Navarrete, M.Á.; Ángeles-Castellanos, M.; Morales-Pérez, B.; Buijs, R.M. Chocolate for breakfast prevents circadian desynchrony in experimental models of jet-lag and shift-work. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Retana-Márquez, S.; Bonilla-Jaime, H.; Vázquez-Palacios, G.; Domínguez-Salazar, E.; Martínez-García, R.; Velázquez-Moctezuma, J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology 2003, 28, 207–227. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, L.J.; Xu, L.Z.; Guo, T.Y.; Yue, X.D.; Li, S.X. Effects of environmental stress on the depression-like behaviors and the diurnal rhythm of corticosterone and melatonin in male rats-PubMed. Sheng Li Xue Bao 2016, 68, 215–223. [Google Scholar] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pons, Z.; Guerrero, L.; Margalef, M.; Arola, L.; Arola-Arnal, A.; Muguerza, B. Effect of low molecular grape seed proanthocyanidins on blood pressure and lipid homeostasis in cafeteria diet-fed rats. J. Physiol. Biochem. 2014, 70, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Margalef, M.; Pons, Z.; Carres, L.I.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A. Flavanol plasma bioavailability is affected by metabolic syndrome in rats. Food Chem. 2017, 231, 287–294. [Google Scholar] [CrossRef]
- Margalef, M.; Pons, Z.; Carres, L.I.; Quiñones, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Rat health status affects bioavailability, target tissue levels, and bioactivity of grape seed flavanols. Mol. Nutr. Food Res. 2016, 61. [Google Scholar] [CrossRef]
- Escobar-Martínez, I.; Arreaza-Gil, V.; Muguerza, B.; Arola-Arnal, A.; Bravo, F.I.; Torres-Fuentes, C.; Suárez, M. Administration Time Significantly Affects Plasma Bioavailability of Grape Seed Proanthocyanidins Extract in Healthy and Obese Fischer 344 Rats. Mol. Nutr. Food Res. 2021, 66, 2100552. [Google Scholar] [CrossRef]
- Carres, L.I.; Mas-Capdevila, A.; Bravo, F.I.; Arola, L.; Muguerza, B.; Arola-Arnal, A. Exposure of Fischer 344 rats to distinct photoperiods influences the bioavailability of red grape polyphenols. J. Photochem. Photobiol. B Biol. 2019, 199, 111623. [Google Scholar] [CrossRef]
- Ibars, M.; Aragonès, G.; Ardid-Ruiz, A.; Ramos, A.G.; Arola-Arnal, A.; Suárez, M.; Bladé, C. Seasonal consumption of polyphenol-rich fruits affects the hypothalamic leptin signaling system in a photoperiod-dependent mode. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Caimari, A.; Arola, L. Cherry consumption out of season alters lipid and glucose homeostasis in normoweight and cafeteria-fed obese Fischer 344 rats. J. Nutr. Biochem. 2019, 63, 72–86. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Crescenti, A.; Salvadó, M.J. Consumption of cherry out of season changes white adipose tissue gene expression and morphology to a phenotype prone to fat accumulation. Nutrients 2018, 10, 1102. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Carrión, Á.; De Azua, M.J.R.; Mulero, M.; Arola-Arnal, A.; Suárez, M. Oxidative Stress in Rats is Modulated by Seasonal Consumption of Sweet Cherries from Different Geographical Origins: Local vs. Non-Local. Nutrients 2020, 12, 2854. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Bautista, E.; Velasco-Ramos, M.; Osnaya-Ramírez, I.; Ángeles-Castellanos, M.; Buijs, R.M.; Escobar, C. Social jet-lag potentiates obesity and metabolic syndrome when combined with cafeteria diet in rats. Metab.-Clin. Exp. 2017, 72, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Mariné-Casadó, R.; Domenech-Coca, C.; Del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. Intake of an obesogenic cafeteria diet affects body weight, feeding behavior, and glucose and lipid metabolism in a photoperiod-dependent manner in F344 rats. Front. Physiol. 2018, 9, 1639. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Casanova-Martí, A.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Z. Für. Ernährungswissenschaft 2017, 56, 1629–1636. [Google Scholar] [CrossRef]
- Caimari, A.; Mariné-Casadó, R.; Boqué, N.; Crescenti, A.; Arola, L.; Del Bas, J.M. Maternal intake of grape seed procyanidins during lactation induces insulin resistance and an adiponectin resistance-like phenotype in rat offspring. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J. The Pineal and Its Hormones in the Control of Reproduction in Mammals. Endocr. Rev. 1980, 1, 109–131. [Google Scholar] [CrossRef]
- Robinson, I.; Reddy, A. Molecular mechanisms of the circadian clockwork in mammals. FEBS Lett. 2014, 588, 2477–2483. [Google Scholar] [CrossRef]
- Badness, T.J.; Powers, J.B.; Hastings, M.H.; Bittman, E.L.; Goldman, B.D. The timed infusion paradigm for melatonin delivery: What has it taught us about the melatonin signal, its reception, and the photoperiodic control of seasonal responses? J. Pineal Res. 1993, 15, 161–190. [Google Scholar] [CrossRef]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Wiley, J.W.; Higgins, G.; Athey, B.D. Stress and glucocorticoid receptor transcriptional programming in time and space: Implications for the brain-gut axis. Neurogastroenterol. Motil. 2016, 28, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Charmandari, E. Primary Generalized Glucocorticoid Resistance and Hypersensitivity. Horm. Res. Paediatr. 2011, 76, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.-W.; Blum, I.D.; Storch, K.-F. Clocks within the Master Gland. J. Biol. Rhythm. 2015, 30, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Domenech-Coca, C.; Mariné-Casadó, R.; Caimari, A.; Arola, L.; del Bas, J.M.; Bladé, C.; Rodriguez-Naranjo, M.I. Dual liquid-liquid extraction followed by LC-MS/MS method for the simultaneous quantification of melatonin, cortisol, triiodothyronine, thyroxine and testosterone levels in serum: Applications to a photoperiod study in rats. J. Chromatogr. B 2019, 1108, 11–16. [Google Scholar] [CrossRef]
- Dorgan, J.F.; Judd, J.T.; Longcope, C.; Brown, C.; Schatzkin, A.; Clevidence, B.A.; Campbell, W.S.; Nair, P.P.; Franz, C.; Kahle, L.; et al. Effects of dietary fat and fiber on plasma and urine androgens and estrogens in men: A controlled feeding study. Am. J. Clin. Nutr. 1996, 64, 850–855. [Google Scholar] [CrossRef]
- Whittaker, J.; Wu, K. Low-fat diets and testosterone in men: Systematic review and meta-analysis of intervention studies. J. Steroid Biochem. Mol. Biol. 2021, 210, 105878. [Google Scholar] [CrossRef]
- Cokelaere, M.; Decuypere, E.; Flo, G.; Darras, V.; Kühn, E. Influence of feeding pattern on thyroid hormones in long-term food-restricted rats. Horm. Metab. Res. 1996, 28, 315–318. [Google Scholar] [CrossRef]
- Nimalasuriya, A.; Spencer, C.A.; Lin, S.C.; Tse, J.K.; Nicoloff, J.T. Studies on the Dirunal Pattern of Serum 3,5,3′-Triiodothyronine. J. Clin. Endocrinol. Metab. 1986, 62, 153–158. [Google Scholar] [CrossRef]






| Diet | L12 Cumulative 6 Weeks | t-Student | Photoperiod | VH 7th Week | GSPE 7th Week | ANOVA | |
|---|---|---|---|---|---|---|---|
| Food Intake (g) | STD | 120.21 (±3.41) | p < 0.001 | L6 | 19.69 (±0.97) | 22.61 (±1.44) | D |
| L18 | 19.23 (±0.82) | 20.05 (±0.80) | |||||
| CAF | 321.81 (±6.98) | L6 | 57.99 (±3.79) * | 57.71 (±2.30) * | |||
| L18 | 48.93 (±5.64) * | 51.94 (±4.61) * | |||||
| Energy Intake (Kcal) | STD | 419.70 (±8.25) | p < 0.001 | L6 | 65.76 (±3.24) | 75.51 (±4.81) | D |
| L18 | 64.22 (±2.74) | 66.97 (±2.68) | |||||
| CAF | 1335.17 (±28.45) | L6 | 233.08 (±18.34) * | 233.64 (±10.32) * | |||
| L18 | 192.59 (±31.13) * | 216.21 (±27.48) * | |||||
| Energy intake from Protein (Kcal) | STD | 79.74 (±1.57) | p < 0.001 | L6 | 12.50 (±0.62) | 14.35 (±0.91) & | D |
| L18 | 12.20 (±0.52) | 12.72 (±0.51) | |||||
| CAF | 119.62 (±1.38) | L6 | 16.06 (±0.76) * | 17.27 (±0.43) * | |||
| L18 | 16.52 (±0.65) * | 17.78 (±1.33) * | |||||
| Energy intake from Carbohydrates (Kcal) | STD | 302.19 (±5.94) | p < 0.001 | L6 | 47.35 (±2.33) | 54.36 (±3.46) | D, P |
| L18 | 46.24 (±1.97) | 48.22 (±1.93) | |||||
| CAF | 911.50 (±28.22) | L6 | 179.12 (±15.87) * | 172.16 (±10.30) * | |||
| L18 | 138.59 (±27.29) *,# | 145.83 (±26.77) * | |||||
| Energy intake from Fat (Kcal) | STD | 33.58 (±0.66) | p < 0.001 | L6 | 5.26 (±0.26) | 6.04 (±0.38) | D, T, DxT, DxP |
| L18 | 5.14 (±0.22) | 5.36 (±0.21) | |||||
| CAF | 416.76 (±7.35) | L6 | 50.04 (±3.57) * | 57.08 (±1.23) *,$ | |||
| L18 | 52.80 (±3.76) * | 66.81 (±4.67) *,$,# | |||||
| AUC Energy from carbohydrates oxidation | STD | 75,639.10 (±9703.77) | p = 0.02 | L6 | 100,366 (±21,217) | 112,676 (±11,496) | D |
| L18 | 112,095 (±11,294) | 106,221 (±5877) | |||||
| CAF | 49,412.4 (±6075.05) | L6 | 71,406 (±9041) * | 67,067 (±14,008) * | |||
| L18 | 69,592 (±6221) * | 35,767 (±11,233) *,$ | |||||
| AUC Energy from fat oxidation | STD | 111,461 (±11,654) | p = 0.008 | L6 | 64,388 (±16,072) | 48,017 (±11,396) | D |
| L18 | 50,167 (±11,689) | 48,442 (±2447) | |||||
| CAF | 165,171 (±15,493) | L6 | 97,490 (±9362) * | 94,484 (±16,432) * | |||
| L18 | 130,001 (±22,824) * | 122,074 (±16,528) * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliz-Rueda, J.R.; López-Fernández-Sobrino, R.; Bravo, F.I.; Aragonès, G.; Suarez, M.; Muguerza, B. Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats. Nutrients 2022, 14, 1834. https://doi.org/10.3390/nu14091834
Soliz-Rueda JR, López-Fernández-Sobrino R, Bravo FI, Aragonès G, Suarez M, Muguerza B. Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats. Nutrients. 2022; 14(9):1834. https://doi.org/10.3390/nu14091834
Chicago/Turabian StyleSoliz-Rueda, Jorge R., Raúl López-Fernández-Sobrino, Francisca Isabel Bravo, Gerard Aragonès, Manuel Suarez, and Begoña Muguerza. 2022. "Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats" Nutrients 14, no. 9: 1834. https://doi.org/10.3390/nu14091834
APA StyleSoliz-Rueda, J. R., López-Fernández-Sobrino, R., Bravo, F. I., Aragonès, G., Suarez, M., & Muguerza, B. (2022). Grape Seed Proanthocyanidins Mitigate the Disturbances Caused by an Abrupt Photoperiod Change in Healthy and Obese Rats. Nutrients, 14(9), 1834. https://doi.org/10.3390/nu14091834