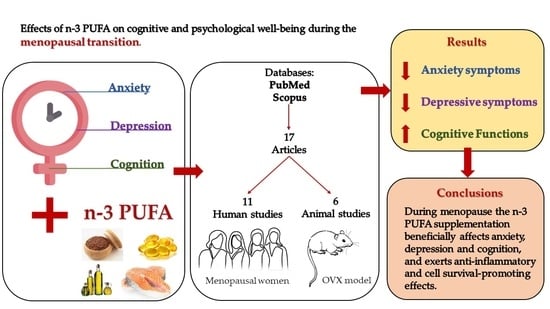
n-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review
Abstract
:1. Introduction
1.1. Menopause as a Delicate Phase of Women Life
- Menopause: “the permanent cessation of menstruation resulting from loss of ovarian follicular activity”;
- Perimenopause (or climacteric or menopausal transition): “the period immediately prior to the menopause (when the endocrinological, biological and clinical features of approaching menopause commence) and at least the first year after the menopause”;
- Postmenopause: the period “from the menopause, although it cannot be determined until after a period of 12 months of spontaneous amenorrhea has been observed”.
- -
- Mind-body practices: hypnosis, cognitive-behavioral therapy, relaxation, biofeedback, meditation, and aromatherapy, mindfulness;
- -
- The use of natural products: herbs, vitamins, minerals, and dietary supplements.
1.2. n-3 PUFA Dietary Intake
2. Materials and Methods
2.1. Protocol
2.2. Search Strategy and Study Selection
2.3. Inclusion and Exclusion Criteria
- -
- P (population): “women in menopausal transition and ovariectomized rodents”;
- -
- I (intervention): “n-3 PUFA dietary intake and n-3 PUFA supplementation”;
- -
- C (comparators): “control group and placebo”;
- -
- O (outcome): “emotional and cognitive outcomes”;
- -
- S (study design): “observational studies, clinical and preclinical trials”.
- -
- Anxiety;
- -
- Depression;
- -
- Cognition.
2.4. Data Extraction
3. Results
3.1. Selected Studies
3.2. Effects of n-3 PUFA on Anxiety
3.3. Effects of n-3 PUFA on Depression
3.4. Effects of n-3 PUFA on Cognition
4. Discussion
4.1. n-3 PUFA and Anxiety
4.2. n-3 PUFA and Depression
4.3. n-3 PUFA and Cognition
4.4. n-3 PUFA and Biochemical Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brown, J.B. Types of ovarian activity in women and their significance: The continuum (a reinterpretation of early findings). Hum. Reprod. 2011, 17, 141–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brann, D.W.; Dhandapani, K.; Wakade, C.; Mahesh, V.B.; Khan, M.M. Neurotrophic and neuroprotective actions of estrogen: Basic mechanisms and clinical implications. Steroids 2007, 72, 381–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krolick, K.N.; Zhu, Q.; Shi, H. Effects of Estrogens on Central Nervous System Neurotransmission: Implications for Sex Differences in Mental Disorders. Prog. Mol. Biol. Transl. Sci. 2018, 160, 105–171. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Invited review: Estrogen effects on the brain: Multiple sites and molecular mechanisms. J. Appl. Physiol. 2001, 91, 2785–2801. [Google Scholar] [CrossRef]
- Morgan, K.N.; Derby, C.A.; Gleason, C.E. Cognitive changes with reproductive aging, perimenopause, and menopause. Obstet. Gynecol. Clin. N. Am. 2018, 45, 751–763. [Google Scholar] [CrossRef]
- World Health Organization. IRIS (Institutional Repository for Information Sharing). Available online: https://apps.who.int/iris/handle/10665/41526 (accessed on 5 April 2022).
- Gava, G.; Orsili, I.; Alvisi, S.; Mancini, I.; Seracchioli, R.; Meriggiola, M.C. Cognition, mood and sleep in menopausal transition: The role of menopause hormone therapy. Medicina 2019, 55, 668. [Google Scholar] [CrossRef] [Green Version]
- Burger, H.G.; Cahir, N.; Robertson, D.M.; Groome, N.P.; Dudley, E.; Green, A.; Dennerstein, L. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin. Endocrinol. 1998, 48, 809–813. [Google Scholar] [CrossRef]
- Burger, H.G.; Hale, G.E.; Dennerstein, L.; Robertson, D.M. Cycle and hormone changes during perimenopause: The key role of ovarian function. Menopause 2008, 15, 603–612. [Google Scholar] [CrossRef]
- Guérin, E.; Prud’Homme, D.; Goldfield, G. Trajectories of mood and stress and relationships with protective factors during the transition to menopause: Results using latent class growth modeling in a Canadian cohort. Arch. Women’s Ment. Health 2017, 20, 733–745. [Google Scholar] [CrossRef]
- Rubinow, D.R.; Johnson, S.L.; Schmidt, P.J.; Girdler, S.; Gaynes, B. Efficacy of estradiol in perimenopausal depression: So much promise and so few answers. Depress. Anxiety 2015, 32, 539–549. [Google Scholar] [CrossRef]
- Soares, C.N. Depression and menopause: An update on current knowledge and clinical management for this critical window. Med. Clin. N. Am. 2019, 103, 651–667. [Google Scholar] [CrossRef]
- Mattina, G.F.; Van Lieshout, R.J.; Steiner, M. Inflammation, depression and cardiovascular disease in women: The role of the immune system across critical reproductive events. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719851950. [Google Scholar] [CrossRef]
- Amin, Z.; Canli, T.; Epperson, C.N. Effect of estrogen-serotonin interactions on mood and cognition. Behav. Cogn. Neurosci. Rev. 2005, 4, 43–58. [Google Scholar] [CrossRef]
- Sullivan Mitchell, E.; Fugate Woods, N. Midlife women’s attributions about perceived memory changes: Observations from the Seattle Midlife Women’s Health Study. J. Womens Health Gend. Based Med. 2001, 10, 351–362. [Google Scholar] [CrossRef]
- Pertesi, S.; Coughlan, G.; Puthusseryppady, V.; Morris, E.; Hornberger, M. Menopause, cognition and dementia—A review. Post. Reprod. Health 2019, 25, 200–206. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Scrimo, P.J.; Merchenthaler, I. Estrogen binding and estrogen receptor characterization (ERalpha and ERbeta) in the cholinergic neurons of the rat basal forebrain. Neuroscience 2000, 96, 41–49. [Google Scholar] [CrossRef]
- Maki, P.M. Estrogen effects on the hippocampus and frontal lobes. Int. J. Fertil. Womens Med. 2005, 50, 67–71. [Google Scholar]
- Shanmugan, S.; Epperson, C.N. Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum. Brain Mapp. 2014, 35, 847–865. [Google Scholar] [CrossRef] [Green Version]
- Barha, C.K.; Galea, L.A. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim. Biophys. Acta 2010, 1800, 1056–1067. [Google Scholar] [CrossRef]
- Nelson, H.D. Menopause. Lancet 2008, 371, 760–770. [Google Scholar] [CrossRef]
- Parazzini, F.; Progetto Menopausa Italia Study Group. Determinants of age at menopause in women attending menopause clinics in Italy. Maturitas 2007, 56, 280–287. [Google Scholar] [CrossRef]
- Gold, E.B.; Bromberger, J.; Crawford, S.; Samuels, S.; Greendale, G.A.; Harlow, S.D.; Skurnick, J. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am. J. Epidemiol. 2001, 153, 865–874. [Google Scholar] [CrossRef] [Green Version]
- Melby, M.K.; Lock, M.; Kaufert, P. Culture and symptom reporting at menopause. Hum. Reprod. Update 2005, 11, 495–512. [Google Scholar] [CrossRef] [Green Version]
- Hunter, M.; Rendall, M. Bio-psycho-socio-cultural perspectives on menopause. Best practice & research. Clin. Obstet. Gynecol. 2007, 21, 261–274. [Google Scholar] [CrossRef]
- Schneider, H.P.G.; Birkhäuser, M. Quality of life in climacteric women. Climacteric 2017, 20, 187–194. [Google Scholar] [CrossRef]
- Koebele, S.V.; Bimonte-Nelson, H.A. Modeling menopause: The utility of rodents in translational behavioral endocrinology research. Maturitas 2016, 87, 5–17. [Google Scholar] [CrossRef] [Green Version]
- Diaz Brinton, R. Minireview: Translational animal models of human menopause: Challenges and emerging opportunities. Endocrinology 2013, 153, 3571–3578. [Google Scholar] [CrossRef]
- Medina-Contreras, J.; Villalobos-Molina, R.; Zarain-Herzberg, A.; Balderas-Villalobos, J. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol. Cell. Biochem. 2020, 475, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Henderson, V.W. Cognition and the menopause transition. Menopause 2016, 23, 803–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Drieling, R.; Stafford, R.S. US women desire greater professional guidance on hormone and alternative therapies for menopause symptom management. Menopause 2006, 13, 506–516. [Google Scholar] [CrossRef]
- Posadzki, P.; Lee, M.S.; Moon, T.W.; Choi, T.Y.; Park, T.Y.; Ernst, E. Prevalence of complementary and alternative medicine (CAM) use by menopausal women: A systematic review of surveys. Maturitas 2013, 75, 34–43. [Google Scholar] [CrossRef]
- Johnson, A.; Roberts, L.; Elkins, G. Complementary and alternative medicine for menopause. J. Evid. Based. Integr. Med. 2019, 24, 2515690X19829380. [Google Scholar] [CrossRef] [Green Version]
- Su, H.M. Mechanisms of n-3 fatty acid-mediated development and maintenance of learning memory performance. J. Nutr. Biochem. 2010, 21, 364–373. [Google Scholar] [CrossRef]
- Denis, I.; Potier, B.; Vancassel, S.; Heberden, C.; Lavialle, M. Omega-3 fatty acids and brain resistance to ageing and stress: Body of evidence and possible mechanisms. Ageing Res. Rev. 2013, 12, 579–594. [Google Scholar] [CrossRef]
- Luchtman, D.W.; Song, C. Cognitive enhancement by omega-3 fatty acids from child-hood to old age: Findings from animal and clinical studies. Neuropharmacology 2013, 64, 550–565. [Google Scholar] [CrossRef]
- Denis, I.; Potier, B.; Heberden, C.; Vancassel, S. Omega-3 polyunsaturated fatty acids and brain aging. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.W. Omega-3 fatty acids and mental health. J. Glob. Health 2020, 4, 18–30. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [Green Version]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef] [Green Version]
- De Roos, B.; Mavrommatis, Y.; Brouwer, I.A. Long-chain n-3 polyunsaturated fatty acids: New insights into mechanisms relating to inflammation and coronary heart disease. Br. J. Pharmacol. 2009, 158, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Wiktorowska-Owczarek, A.; Berezińska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Tocher, D.R.; Betancor, M.B.; Sprague, M.; Olsen, R.E.; Napier, J.A. Omega-3 long-chain polyunsaturated fatty acids, EPA and DHA: Bridging the gap between supply and demand. Nutrients 2019, 11, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mohammady, M.; Janani, L.; Jahanfar, S.; Mousavi, M.S. Effect of omega-3 supplements on vasomotor symptoms in postmenopausal women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food. Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Pusceddu, M.M.; Kelly, P.; Ariffin, N.; Cryan, J.F.; Clarke, G.; Dinan, T.G. n-3 PUFAs have beneficial effects on anxiety and cognition in female rats: Effects of early life stress. Psychoneuroendocrinology 2015, 58, 79–90. [Google Scholar] [CrossRef]
- Carney, R.M.; Steinmeyer, B.C.; Freedland, K.E.; Rubin, E.H.; Rich, M.W.; Harris, W.S. Baseline blood levels of omega-3 and depression remission: A secondary analysis of data from a placebo-controlled trial of omega-3 supplements. J. Clin. Psychiatry 2016, 77, e138–e143. [Google Scholar] [CrossRef] [Green Version]
- Dangour, A.D.; Allen, E.; Elbourne, D.; Fasey, N.; Fletcher, A.E.; Hardy, P.; Holder, G.E.; Knight, R.; Letley, L.; Richards, M.; et al. Effect of 2-y n-3 long-chain polyunsaturated fatty acid supplementation on cognitive function in older people: A randomized, double-blind, controlled trial. Am. J. Clin. Nutr. 2010, 91, 1725–1732. [Google Scholar] [CrossRef] [Green Version]
- Ubeda, N.; Achón, M.; Varela-Moreiras, G. Omega 3 fatty acids in the elderly. Br. J. Nutr. 2012, 107, S137–S151. [Google Scholar] [CrossRef] [Green Version]
- Barberger-Gateau, P.; Raffaitin, C.; Letenneur, L.; Berr, C.; Tzourio, C.; Dartigues, J.F.; Alpérovitch, A. Dietary patterns and risk of dementia: The Three-City cohort study. Neurology 2007, 69, 1921–1930. [Google Scholar] [CrossRef]
- Vines, A.; Delattre, A.M.; Lima, M.M.; Rodrigues, L.S.; Suchecki, D.; Machado, R.B.; Tufik, S.; Pereira, S.I.; Zanata, S.M.; Ferraz, A.C. The role of 5-HT1A receptors in fish oil-mediated increased BDNF expression in the rat hippocampus and cortex: A possible antidepressant mechanism. Neuropharmacology 2012, 62, 1841–1891. [Google Scholar] [CrossRef]
- Vancassel, S.; Leman, S.; Hanonick, L.; Denis, S.; Roger, J.; Nollet, M.; Bodard, S.; Kousignian, I.; Belzung, C.; Chalon, S. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. J. Lipid Res. 2008, 49, 340–348. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef] [Green Version]
- Dyall, S.C.; Michael, G.J.; Michael-Titus, A.T. Omega-3 fatty acids reverse age-related decreases in nuclear receptors and increase neurogenesis in old rats. J. Neurosci. Res. 2010, 88, 2091–2102. [Google Scholar] [CrossRef]
- Song, C.; Leonard, B.E.; Horrobin, D.F. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress 2004, 7, 43–54. [Google Scholar] [CrossRef]
- Ferraz, A.C.; Delattre, A.M.; Almendra, R.G.; Sonagli, M.; Borges, C.; Araujo, P.; Andersen, M.L.; Tufik, S.; Lima, M.M. Chronic ω-3 fatty acids supplementation promotes beneficial effects on anxiety, cognitive and depressive-like behaviors in rats subjected to a restraint stress protocol. Behav. Brain Res. 2011, 219, 116–122. [Google Scholar] [CrossRef]
- Farooqui, A.A.; Horrocks, L.A.; Farooqui, T. Modulation of inflammation in brain: A matter of fat. J. Neurochem. 2007, 101, 577–599. [Google Scholar] [CrossRef]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: Preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Saaiq, M.; Ashraf, B. Modifying «Pico» Question into «Picos» model for more robust and reproducible presentation of the methodology employed in a scientific study. World J. Plast. Surg. 2017, 6, 390–392. [Google Scholar]
- Dornellas, A.P.; Boldarine, V.T.; Pedroso, A.P.; Carvalho, L.O.; De Andrade, I.S.; Vulcani-Freitas, T.M.; Dos Santos, C.C.; do Nascimento, C.M.; Oyama, L.M.; Ribeiro, E.B. High-fat feeding improves anxiety-type behavior induced by ovariectomy in rats. Front. Neurosci. 2018, 12, 557. [Google Scholar] [CrossRef]
- Wu, B.; Song, Q.; Zhang, Y.; Wang, C.; Yang, M.; Zhang, J.; Han, W.; Jiang, P. Antidepressant activity of ω-3 polyunsaturated fatty acids in ovariectomized rats: Role of neuroinflammation and microglial polarization. Lipids Health Dis. 2020, 19, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Rocha, R.V.O.; Martins, M.I.M.; Antunes, F.T.T.; Martins, M.G.; Klein, A.B.; Corrêa, D.S.; de Souza, A.H. Behavioral, Oxidative, and Biochemical Effects of Omega-3 on an Ovariectomized Rat Model of Menopause. J. Menopausal Med. 2021, 27, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Park, Y. N-3 polyunsaturated fatty acids and 17β-estradiol injection induce antidepressant-like effects through regulation of serotonergic neurotransmission in ovariectomized rats. J. Nutr. Biochem. 2015, 26, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.E.; Park, Y. EPA and DHA; but not ALA; have antidepressant effects with 17β-estradiol injection via regulation of a neurobiological system in ovariectomized rats. J. Nutr. Biochem. 2017, 49, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Konuri, A.; Bhat, K.M.; Rai, K.S.; Gourishetti, K.; Phaneendra, M. Supplementation of fenugreek with choline–docosahexaenoic acid attenuates menopause induced memory loss; BDNF and dendritic arborization in ovariectomized rats. Anat. Sci. Int. 2021, 96, 197–211. [Google Scholar] [CrossRef]
- Cohen, L.S.; Joffe, H.; Guthrie, K.A.; Ensrud, K.E.; Freeman, M.; Carpenter, J.S.; Learman, L.A.; Newton, K.M.; Reed, S.D.; Manson, J.E.; et al. Efficacy of omega-3 treatment for vasomotor symptoms: A randomized controlled trial: Omega-3 treatment for vasomotor symptoms. Menopause 2014, 21, 347. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Asselin, G.; Mérette, C.; Poulin, M.J.; Dodin, S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: A double-blind; placebo-controlled; randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Freeman, M.P.; Hibbeln, J.R.; Silver, M.; Hirschberg, A.M.; Wang, B.; Yule, A.M.; Petrillo, L.F.; Pascuillo, E.; Economou, N.I.; Joffe, H.; et al. Omega-3 fatty acids for major depressive disorder associated with the menopausal transition: A preliminary open trial. Menopause 2011, 18, 279. [Google Scholar] [CrossRef] [Green Version]
- Persons, J.E.; Robinson, J.G.; Ammann, E.M.; Coryell, W.H.; Espeland, M.A.; Harris, W.S.; Manson, J.E.; Fiedorowicz, J.G. Omega-3 fatty acid biomarkers and subsequent depressive symptoms. Int. J. Geriatr. Psychiatry 2014, 29, 747–757. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Kim, T.H.; Park, Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause 2016, 23, 1012–1018. [Google Scholar] [CrossRef]
- Masoumi, S.Z.; Kazemi, F.; Tavakolian, S.; Rahimi, A.; Oshvandi, K.; Soltanian, A.; Shobeiri, F. Effect of citalopram in combination with omega-3 on depression in post-menopausal women: A triple blind randomized controlled trial. J. Clin. Diagn. Res. 2016, 10, QC01. [Google Scholar] [CrossRef]
- Colangelo, L.A.; Ouyang, P.; Golden, S.H.; Szklo, M.; Gapstur, S.M.; Vaidya, D.; Liu, K. Do sex hormones or hormone therapy modify the relation of n-3 fatty acids with incident depressive symptoms in postmenopausal women? The MESA Study. Psychoneuroendocrinology 2017, 75, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Liang, H.; Tong, Y.; Li, Y. Association of dietary n-3 polyunsaturated fatty acids intake with depressive symptoms in midlife women. J. Affect. Disord. 2020, 261, 164–171. [Google Scholar] [CrossRef]
- Chae, M.; Park, K. Association between dietary omega-3 fatty acid intake and depression in postmenopausal women. Nutr. Res. Pract. 2021, 15, 468–478. [Google Scholar] [CrossRef]
- Ammann, E.M.; Pottala, J.V.; Harris, W.S.; Espeland, M.A.; Wallace, R.; Denburg, N.L.; Carnahan, R.M.; Robinson, J.G. Omega-3 fatty acids and domain-specific cognitive aging: Secondary analyses of data from WHISCA. Neurology 2013, 81, 1484–1491. [Google Scholar] [CrossRef] [Green Version]
- Strike, S.C.; Carlisle, A.; Gibson, E.L.; Dyall, S.C. A high omega-3 fatty acid multinutrient supplement benefits cognition and mobility in older women: A randomized; double-blind; placebo-controlled pilot study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 236–242. [Google Scholar] [CrossRef] [Green Version]
- De la Presa Owens, S.; Innis, S.M. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and alpha-linolenic acid deficient diet in formula-fed piglets. J. Nutr. 1999, 129, 2088–2093. [Google Scholar] [CrossRef] [Green Version]
- Chalon, S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 259–269. [Google Scholar] [CrossRef]
- McNamara, R.K.; Able, J.; Liu, Y.; Jandacek, R.; Rider, T.; Tso, P.; Lipton, J.W. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: Dissociation from estrogenic effects. J. Psychiatr. Res. 2009, 43, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Lakhwani, L.A.L.I.T.; Tongia, S.K.; Pal, V.S.; Agrawal, R.P.; Nyati, P.R.E.M.; Phadnis, P.R.A.D.E.E.P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Pol. Pharm. 2007, 64, 271–276. [Google Scholar]
- Arbabi, L.; Baharuldin, M.T.H.; Moklas, M.A.M.; Fakurazi, S.; Muhammad, S.I. Antidepressant-like effects of omega-3 fatty acids in postpartum model of depression in rats. Behav. Brain Res. 2014, 271, 65–71. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Sites, C.K.; Toth, M.J.; Cushman, M.; L’Hommedieu, G.D.; Tchernof, A.; Tracy, R.P.; Poehlman, E.T. Menopause-related differences in inflammation markers and their relationship to body fat distribution and insulin-stimulated glucose disposal. Fertil. Steril. 2002, 77, 128–135. [Google Scholar] [CrossRef]
- Gameiro, C.M.; Romão, F.; Castelo-Branco, C. Menopause and aging: Changes in the immune system-a review. Maturitas 2010, 67, 316–320. [Google Scholar] [CrossRef]



| Article | Main Topic | Study Design | Age-Strain | Ovariectomy | n-3 PUFA Treatment Details |
|---|---|---|---|---|---|
| Dornellas et al. [64] | Anxiety and Depression | Preclinical study | 8 weeks old; Wistar Rats | X | Fish Diet: standard chow enriched with fish oil. The high-fat diets were prepared by adding, to the standard chow, 20% (w/w) fish oil, 20% (w/w) casein, 10% (w/w) sucrose, and 0.02% (w/w) butylated hydroxytoluene; duration: 8 weeks. |
| Wu et al. [65] | Anxiety and Depression | Preclinical study | 12 weeks old; Sprague Dawley Rats | X | Refined fish oil administrated daily by gavage (1.5 g/kg, approximately 340 mg/g for EPA, 240 mg/g for DHA); duration: 10 weeks. |
| Da Rocha et al. [66] | Anxiety and Depression | Preclinical study | 8 weeks old; Wistar Rats | X | Supplementation performed 20 days before and 20 days after the surgical procedure: daily dose of 500 mg/kg/day of omega-3 (1000 mg capsules, containing 180 mg of EPA and 120 mg of DHA); duration: 40 days. |
| Jin et al. [67] | Depression | Preclinical study | 3 weeks old; Wistar Rats | X (after 8 weeks of supplementation) | The diets were isocaloric and modified with 0%, 1% or 2% EPA + DHA relative to the total energy intake (0 g, 8.09 g and 16.21 g of fish oil per kg of diet, respectively). The diets contained 10% of total energy from fat, with 42.94 g/kg diet of fish oil and grape seed oil; duration: 12 weeks. |
| Choi et al. [68] | Depression | Preclinical study | 3 weeks old; Wistar Rats | X (after 8 weeks of supplementation) | Diets were isocaloric modified with 0% n-3 PUFA, 1% ALA, 1% EPA or 1% DHA relative to the total energy intake. The control diet contained 70 g/kg of grape seed oil; the ALA, EPA and DHA diets contained 8.27 g/kg of flaxseed oil, 4.46 g/kg of EPA EE and 4.67 g/kg of DHA EE, respectively. The control diet had 0.05% of n-3 PUFA, and the ALA, EPA and DHA diets had 6.40% of ALA, 6.35% of EPA and 6.35% of DHA in total fatty acids, respectively; duration: 12 weeks. |
| Konuri et al. [69] | Cognition | Preclinical study | 9–10 months; Wistar Rats | X | Choline (4.6 mmol/kg/day) in combination with DHA (300 mg/kg/day); duration: 30 days. |
| Article | Behavioral Tests | Biochemical Analyses | Behavioral Results | Biochemical Results |
|---|---|---|---|---|
| Dornellas et al. [64] | EPM; FST Modified. | No Biochemical Analyses were performed. | High fat diet had an anxiolytic effect regardless the fatty acid composition. No differences were found in depressive-like behaviors. | In the hippocampus, fish oil diet induced a stimulation in the serotoninergic activity, which is expressed in an increase in 5-hydroxyindoleacetic acid levels and in serotonergic turnover. |
| Wu et al. [65] | EPM; FST; Sucrose Preference Test; Novelty Suppressed Feeding Test. | Hormone Assay: ELISA Kit for E2; Apoptosis analysis: terminal deoxynucleotidyl transferase-mediated FITC-dUTP nick end labelling (Tunel) method; Microglia activation: Immunostaining of Iba-1; Cytokine Expression and Microglia Polarization: Western blot analysis of phosphorylation of NF-κB pp65, p65, IκB, iNOS, Arg-1 and β-actin; RT-PCR analysis. | n-3 PUFA supplementation: reverted the OVX induced anxiety-like behaviors displaying notable anxiolytic properties; alleviated OVX induced depressive-like behaviors in the FST and NSFT. | n-3 PUFA supplementation increased: IL-10; IL-4; IκB; p65. n-3 PUFA supplementation decreased: IL-1β; IL-6; NFκB; n-3 PUFA supplementation ameliorated: microglia activation; neuronal apoptosis. |
| Da Rocha et al. [66] | EPM; FST; Open Field. | Thiobarbituric acid reactive substances and catalase in the brain tissue; Glutamate in the cerebrospinal fluid. | The n-3 PUFA supplementation had an anxiolytic effect increasing the locomotory activity in the OF. The depression-like behavior was improved in the FST. No differences between groups were found in the EPM. | n-3 PUFA supplementation did not had any effect on Thiobarbituric acid reactive substances, catalase and glutamate. |
| Jin et al. [67] | FST. | Gas chromatography for the fatty acid composition of the brain tissue; Brain tissue levels of PGE2; Immunofluorescence staining for ER-α and ER-β; Blood samples collection to measure: serotonin serum levels; plasma estrogen levels; Hippocampal Western blot analysis of: CREB; pCREB; TNF-α; BDNF; IL-1β, IL-6; ER-α or ER-β. | n-3 PUFA supplementation increased climbing and decreased immobility and had no significant effects on duration of swimming. | n-3 PUFA supplementation increased: serum serotonin concentrations; the brain phospholipid level of n-3 PUFA (20:5n3, 22:5n3 and 22:6n3) in a dose-dependent manner; expression of CREB (among 0% vs. 1% and 0% vs. 2%); expression of BDNF (among 0% vs. 2% and 1% vs. 2%); expression of ER-α (among 0% vs. 1% and 0% vs. 2%). n-3 PUFA supplementation decreased: PGE2 brain levels; brain phospholipid level of n-6 PUFA (20:4n6, 22:4n6 and 22:5n6) in a dose-dependent manner; TNF-α (among 0% vs. 2% and 1% vs. 2%); IL-6 (among 0% vs. 1% and 0% vs. 2%). |
| Choi et al. [68] | FST. | Gas chromatography for the fatty acid composition of the brain tissue; Plasma analysis for estrogens and malondialdehyde levels; Brain tissue levels of PGE2; Immunofluorescence staining for BDNF levels in DG. Serum analysis for: serotonin; NOx; superoxide dismutase levels. Hippocampal Western blot analysis for: CREB; pCREB; BDNF; TNF-α; IL-6; ER-α or ER-β. In vivo magnetic resonance imaging/spectroscopy of the left dorsal hippocampal region to calculate peak concentrations of: creatine; phosphocreatine; glucose; glutamate; myo-inositol. | Supplementation with EPA and DHA, but not ALA, decreased the duration of immobility by 49%, and increased climbing by 69%. | Supplementation with: ALA increased brain phospholipid proportion of 18:3n3 as compared to the control, EPA and DHA diet. ALA, EPA and DHA increased the brain phospholipid proportions of 20:5n3, 22:5n3 and 22:6n3, this increase was greater with EPA and DHA than ALA supplementation. ALA, EPA and DHA decreased the brain phospholipid proportions of 18:2n6, 20:4n6, 22:4n6 and 22:5n6 and the decrease of proportions of 20:4n6, 22:4n6 and 22:5n6 were greater with of EPA and DHA than with ALA. EPA and DHA, increase serum serotonin levels by 29%. EPA and DHA decreased: PGE2 brain levels by 37%; serum concentrations of NOx by 52%; TNF-α expression by 26%; IL-6 expression by 29%. EPA and DHA increased hippocampal expression: hippocampal expression of ER-α by 21%; CREB by 34%; pCREB by 56%; BDNF by 32%. |
| Konuri et al. [69] | Eight-arm radial maze test; | Right cerebral hemisphere BDNF analysis using ELISA kit. E2 serum levels measured with ELISA kit. Golgi-Cox staining of the left cerebral hemisphere to evaluate dendritic arborization and length. | The dietary supplementation of choline-DHA significantly improved the memory retention. | The dietary supplementation of choline-DHA: Increase BDNF levels; improved basal and apical dendritic branching points and dendritic intersections in CA1 and CA3; Did not show any effect on serum E2 concentration. |
| Article | MENOPAUSE Definitions |
|---|---|
| Cohen et al. [70] | Menopause transition: amenorrhea ≥60 days in the past year Postmenopause: ≥12 months since last menstrual period or bilateral oophorectomy Hysterectomy: with follicle stimulating hormone >20 mIU/mL and estradiol of ≤50 pg/mL |
| Lucas et al. [71] | Postmenopausal status: 12 months of amenorrhea after the final menstrual period |
| Freeman et al. [72] | Peri- Post- menopause: Women that met perimenopause or postmenopause status as defined by the standardized Stages of Reproductive Aging Workshop criteria |
| Persons et al. [73] | Postmenopause: not specified by authors. |
| Jin et al. [74] | Menopause: not specified by authors. |
| Masoumi et al. [75] | Postmenopause: at least 12 months of amenorrhea. |
| Colangelo et al. [76] | Postmenopause: Women were classified as postmenopausal if (a) they responded ‘yes’ to the question, ‘Have you gone through menopause (change of life)?’, or (b) had a prior hysterectomy and bilateral oophorectomy. |
| Li et al. [77] | Early Perimenopause: menstrual bleeding in the past 3 months accompanied by changes in cycle regularity. Premenopause: menstrual bleeding in the past 3 months with no change in cycle regularity in the past 12 months. |
| Chae and Park [78] | Menopause: not specified by authors. Postmenopause: not specified by authors. |
| Ammann et al. [79] | Postmenopause: not specified by authors. |
| Strike et al. [80] | Postmenopause: not specified by authors. |
| Article | Main Topic | Study Design | Sample Size and Age (Years) | Ethnicity | Exclusion Criteria |
|---|---|---|---|---|---|
| Cohen et al. [70] | Anxiety and Depression | Randomized Controlled Trial | n = 355 Age: 40–62 | White; African American; Other | Body Mass Index > 37; use of hormones or hormonal contraceptives in the past 2 months; use of prescription or over-the-counter treatments for vasomotor symptoms in the past month; any unstable medical conditions; contraindications to exercise training, yoga, or omega-3; current participation in regular exercise or yoga; current use of omega-3 supplements or frequent consumption of fish; MDE in the past three months. |
| Lucas et al. [71] | Depression | Randomized Controlled Trial | n = 120 Age: 40–55 | White | Severe MDE [scores of 26 on HAM-D-21]; history of schizophrenia or bipolar I and II disorder; imminent risk of suicide or homicide; postmenopausal status for >5 years; medical conditions that affect mental health; substance abuse or dependence; fish allergies; high fish consumption (>3 servings/week) in the past 3 months; use of antidepressants; hormone replacement therapy; fish-oil supplements in the past 3 months; anticoagulants use. |
| Freeman et al. [72] | Depression | Open-Label Trial | n = 19 Age mean: 52.5 ± 4.9 | Caucasian; African American; Other | Currently pregnant, breast-feeding, or trying to conceive; currently being treated with an antidepressant, hormone treatment, or n-3 PUFA supplements or with one of the preceding treatments within 1 month of study entry; suicidal ideation; current or recent (past month) diagnosis of panic disorder or obsessive-compulsive disorder or history of psychosis, mania, or hypomania, as assessed by the MINI, diagnosis of treatment-resistant Major Depressive Disorder; fish or fish oil allergies; responded to placebo [950% decrease in the MADRS]. |
| Persons et al. [73] | Depression | Retrospective Cohort Study | n = 7086 Age: 63–81 | Not specified | Not Specified. |
| Jin et al. [74] | Depression | Cross-Sectional Study | n = 214 Age: from 54.23 ± 5.43 to 56.02 ± 6.09 | Koreans | Not Specified. |
| Masoumi et al. [75] | Depression | Randomized Controlled Trial | n = 60 Age: 45–65 | Not specified | Depression scores higher than 30 at follow-ups and any known drug side effects. |
| Colangelo et al. [76] | Depression | Retrospective Cohort Study | n = 1616 Age: 45–84 | Non-Hispanic White; African American; Chinese American; Hispanic | Not Specified. |
| Li et al. [77] | Depression | Cross-Sectional Study | n = 3054 Age: 42–52 | Non-Hispanic White; Chinese; Japanese; Hispanic; Black | No intact uterus or ovaries; use of reproductive hormones and amenorrhea in the previous 3 months. |
| Chae and Park [78] | Depression | Cohort Study | n = 4150 Age: from 62.8 ± 0.3 to 67.1 ± 0.3 | Korean | Men; pregnant, lactating, or premenopausal women; women with a total energy intake of less than 500 kcal or more than 5000 kcal/day; women with no data on depression. |
| Ammann et al. [79] | Cognition | Retrospective Cohort Study | n = 2157 Age: 65–80 | USA | Not Specified. |
| Strike et al. [80] | Cognition | Randomized, Double-Blind, Placebo-Controlled Pilot Study | n = 27 Age: 60–84 | English | Vestibular impairments; neurological disorder; lower limb surgery; allergy to seafood; regular consumption of multivitamin/fish oil supplements. |
| Article | Main Topic | n-3 PUFA Treatment Details | Behavioral Analyses | Biochemical Analyses | Main Results |
|---|---|---|---|---|---|
| Cohen et al. [70] | Anxiety and Depression | 1.8 g/day (3 pills/day, each containing 425 mg of EPA, 100 mg DHA and 90 mg of other omega-3) for 12 weeks. | Physician’s Health Questionnaire-8 (depression domains); Generalized Anxiety Disorder Questionnaire-7. | No Biochemical Analyses were performed. | n-3 PUFA did not improve mood over placebo. |
| Lucas et al. [71] | Depression | 3 capsule/day containing 350 mg EPA and 50 mg DHA in the form of ethyl esters for 8 weeks. | MINI (version 5.0.0); Psychological General Well-Being Schedule; 20-item Hopkins Symptom Checklist Depression Scale; HAM-D-21; Clinical Global Impression Severity Scale; FFQ (based on marine products). | RBCs fatty acid composition. | Ethyl-EPA treatment over placebo improved significantly psychological distress and depressive symptoms in women without MDE. |
| Freeman et al. [72] | Depression | 2 g/day (2 capsules per day each 1-g capsule contains 840 mg of the EE of n-3 PUFA, as a combination of EE of EPA (approximately 465 mg per capsule) and DHA (approximately 375 mg per capsule)) for 8 weeks. | MINI for the diagnosis of Major Depressive Disorder; MADRS. | RBCs fatty acid composition. | Significant decrease in MADRS scores after treatment. |
| Persons et al. [73] | Depression | No treatment has been used in this study. | Burnam 8-item scale for depressive disorders: combined CES-D/DIS short form. | RBCs fatty acid composition. | Positive association between: RBC n-3 PUFA levels (DHA, both EPA + DHA and total n-3 PUFA) and depressive symptoms (the effect disappeared after adjusting data for demographic and health behavior characteristics); n-3 PUFA dietary intake (total n-3 PUFA, DHA, and DHA + EPA) with a higher prevalence of depressive symptoms; the risk to develop depressive symptoms and total n-3 PUFA (in the follow-up analysis, after excluding prevalent cases of depression in baseline). |
| Jin et al. [74] | Depression | No treatment has been used in this study. | BDI; Medical Records to assess at least 3 HT use; Interviews to assess dietary intake and general information. | No Biochemical Analyses were performed. | Significant negative correlation between Erythrocyte levels of n-3 PUFA of ALA, DPA, and DHA and depression only in women using HT. |
| Masoumi et al. [75] | Depression | Citalopram with 1 g of n-3 PUFA for 1 week. | Diagnostic and Statistical Manual of mental disorders-IV questionnaire to assess depression; BDI. | No Biochemical Analyses were performed. | Mean depression score lower in two and four weeks after intervention. |
| Colangelo et al. [76] | Depression | No treatment has been used in this study. | FFQ modified; CES-D. | Blood collection for the assessment of E2. | Significant interaction of HT with n-3 PUFA intake and depressive symptoms. |
| Li et al. [77] | Depression | No treatment has been used in this study. | FFQ; CES-D. | No Biochemical Analyses were performed. | n-3 PUFA intake was negatively correlated with depressive symptoms in early perimenopausal but not in premenopausal women. |
| Chae and Park [78] | Depression | No treatment has been used in this study. | Self-reported mental health questionnaire to assess depression; 24-h phone call interview to assess dietary intake. | No Biochemical Analyses were performed. | n-3 PUFA intake in postmenopausal women was inversely proportional to depression in a dose-response manner. |
| Ammann et al. [79] | Cognition | No treatment has been used in this study. | Finger Tapping Test; Card Rotations Test; Benton Visual Retention Test; California Verbal Learning Test; Primary Mental Abilities (Vocabulary test); Letter and category fluency tests; Digit Span (Forward and Backward Test). | RBCs fatty acid composition. | RBC DHA-EPA levels were not significantly correlated with baseline cognitive function and cognitive change over time. |
| Strike et al. [80] | Cognition | 4 capsules/day (1 g DHA and 160 mg EPA per day in addition to Ginkgo biloba, PS, α-tocopherol, folic acid, and vitamin B12) for 24 weeks. | Cambridge Cognition Ltd.: A battery of computer-based cognitive test; MOT; VRM; Paired Associate Learning; Stockings of Cambridge. | RBCs fatty acid composition. | Supplemented group had: shorter mean latencies in MOT; higher number of words remembered in the VRM. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decandia, D.; Landolfo, E.; Sacchetti, S.; Gelfo, F.; Petrosini, L.; Cutuli, D. n-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review. Nutrients 2022, 14, 1982. https://doi.org/10.3390/nu14091982
Decandia D, Landolfo E, Sacchetti S, Gelfo F, Petrosini L, Cutuli D. n-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review. Nutrients. 2022; 14(9):1982. https://doi.org/10.3390/nu14091982
Chicago/Turabian StyleDecandia, Davide, Eugenia Landolfo, Stefano Sacchetti, Francesca Gelfo, Laura Petrosini, and Debora Cutuli. 2022. "n-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review" Nutrients 14, no. 9: 1982. https://doi.org/10.3390/nu14091982
APA StyleDecandia, D., Landolfo, E., Sacchetti, S., Gelfo, F., Petrosini, L., & Cutuli, D. (2022). n-3 PUFA Improve Emotion and Cognition during Menopause: A Systematic Review. Nutrients, 14(9), 1982. https://doi.org/10.3390/nu14091982