Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach
Abstract
:1. Introduction
1.1. Dietary Patterns and Non-Communicable Diseases
1.2. Biomarkers of Non-Communicable Diseases
1.3. A Role for Network Meta-Analysis
2. Materials and Methods
2.1. Literature Search and Study Selection
2.2. Data Syntheses and Statistical Analyses
3. Results
3.1. Data Availability and Study Characteristics
3.2. Network Meta-Analysis (Dietary Patterns and NCD Biomarkers)
3.2.1. Individual Lipids and Apolipoproteins
3.2.2. Individual Glycemic Biomarkers
3.2.3. Individual Inflammatory Biomarkers
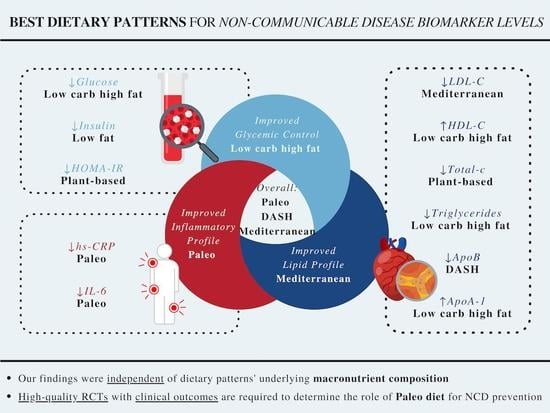
3.2.4. Summary across Outcomes
3.2.5. Inconsistency, Sensitivity Analyses, Publication Bias, and Confidence of Evidence
3.3. Nutritional Geometry Approach (Macronutrients and NCD Biomarkers)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 7 November 2022).
- Institute for Health Metrics and Evaluation. GBD Compare Data Visualization. University of Washington. Available online: https://www.thelancet.com/lancet/visualisations/gbd-compare (accessed on 7 November 2022).
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. 2016, 7, 445–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corella, D.; Ordovas, J.M. Biomarkers: Background, classification and guidelines for applications in nutritional epidemiology. Nutr. Hosp. 2015, 31 (Suppl. S3), 177–188. [Google Scholar] [CrossRef] [PubMed]
- Al-Hadlaq, S.M.; Balto, H.A.; Hassan, W.M.; Marraiki, N.A.; El-Ansary, A.K. Biomarkers of non-communicable chronic disease: An update on contemporary methods. PeerJ 2022, 10, e12977. [Google Scholar] [CrossRef] [PubMed]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef]
- Pirillo, A.; Casula, M.; Olmastroni, E.; Norata, G.D.; Catapano, A.L. Global epidemiology of dyslipidaemias. Nat. Rev. Cardiol. 2021, 18, 689–700. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Langlois, M.R.; Langsted, A.; Chapman, M.J.; Aakre, K.M.; Baum, H.; Borén, J.; Bruckert, E.; Catapano, A.; Cobbaert, C.; et al. Quantifying atherogenic lipoproteins for lipid-lowering strategies: Consensus-based recommendations from EAS and EFLM. Atherosclerosis 2020, 294, 46–61. [Google Scholar] [CrossRef] [Green Version]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, M.B.; Nordestgaard, B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70–100 years: A contemporary primary prevention cohort. Lancet 2020, 396, 1644–1652. [Google Scholar] [CrossRef]
- Farnier, M.; Zeller, M.; Masson, D.; Cottin, Y. Triglycerides and risk of atherosclerotic cardiovascular disease: An update. Arch. Cardiovasc. Dis. 2021, 114, 132–139. [Google Scholar] [CrossRef]
- Singh, B.; Saxena, A. Surrogate markers of insulin resistance: A review. World J. Diabetes 2010, 1, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Rudvik, A.; Månsson, M. Evaluation of surrogate measures of insulin sensitivity—correlation with gold standard is not enough. BMC Med. Res. Methodol. 2018, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C. Dysglycaemia: A cardiovascular risk factor. Diabetes Res. Clin. Pract. 1998, 40, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Antuna-Puente, B.; Disse, E.; Rabasa-Lhoret, R.; Laville, M.; Capeau, J.; Bastard, J.P. How can we measure insulin sensitivity/resistance? Diabetes Metab. 2011, 37, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Lann, D.; LeRoith, D. Insulin Resistance as the Underlying Cause for the Metabolic Syndrome. Med. Clin. N. Am. 2007, 91, 1063–1077. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-González, A.; Rodriguez-Alvarez, M.X.; Gude, F.; García, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Zakai, N.; Katz, R.; Jenny, N.; Psaty, B.; Reiner, A.; Schwartz, S.; Cushman, M. Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: The Cardiovascular Health Study. J. Thromb. Haemost. 2007, 5, 1128–1135. [Google Scholar] [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Liu, C.H.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Hamlet, M.R.J.; Macchiarini, F.; PrabhuDas, M.; Shen, G.L.; Tandon, P.; et al. Biomarkers of chronic inflammation in disease development and prevention: Challenges and opportunities. Nat. Immunol. 2017, 18, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Juber, N.; Lee, C.-C.; Liu, J.J. Associations between high-sensitivity C-reactive protein and non-communicable diseases in an Asian population: Findings from the IFLS study. Biomarkers 2021, 26, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean diet and multiple health outcomes: An umbrella review of meta-analyses of observational studies and randomised trials. Eur. J. Clin. Nutr. 2018, 72, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Piscoya, A.; Marti, K.M.; Marti, K.E.; Pasupuleti, V.; Benites-Zapata, V.A.; Roman, Y.M. Effect of mediterranean diets on cardiovascular risk factors and diseases in the primary prevention setting: A systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 2020, 41. [Google Scholar] [CrossRef]
- Carter, P.; Achana, F.; Troughton, J.; Gray, L.J.; Khunti, K.; Davies, M.J. A Mediterranean diet improves HbA1c but not fasting blood glucose compared to alternative dietary strategies: A network meta-analysis. J. Hum. Nutr. Diet 2014, 27, 280–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Ge, L.; Sadeghirad, B.; Ball, G.D.C.; da Costa, B.R.; Hitchcock, C.L.; Svendrovski, A.; Kiflen, R.; Quadri, K.; Kwon, H.Y.; Karamouzian, M.; et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: Systematic review and network meta-analysis of randomised trials. BMJ 2020, 369, m696. [Google Scholar] [CrossRef] [Green Version]
- Johnston, B.C.; Kanters, S.; Bandayrel, K.; Wu, P.; Naji, F.; Siemieniuk, R.A.; Ball, G.D.; Busse, J.W.; Thorlund, K.; Guyatt, G.; et al. Comparison of weight loss among named diet programs in overweight and obese adults: A meta-analysis. JAMA 2014, 312, 923–933. [Google Scholar] [CrossRef] [Green Version]
- Neuenschwander, M.; Hoffmann, G.; Schwingshackl, L.; Schlesinger, S. Impact of different dietary approaches on blood lipid control in patients with type 2 diabetes mellitus: A systematic review and network meta-analysis. Eur. J. Epidemiol. 2019, 34, 837–852. [Google Scholar] [CrossRef]
- Pan, B.; Wu, Y.; Yang, Q.; Ge, L.; Gao, C.; Xun, Y.; Tian, J.; Ding, G. The impact of major dietary patterns on glycemic control, cardiovascular risk factors, and weight loss in patients with type 2 diabetes: A network meta-analysis. J. Evid. Based Med. 2019, 12, 29–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Punsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2674–2687. [Google Scholar] [CrossRef]
- Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA; U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, S.; Nasir, R.F.; Bell-Anderson, K.S.; Toniutti, C.A.; O’Leary, F.M.; Skilton, M.R. Biomarkers of dietary patterns: A systematic review of randomized controlled trials. Nutr. Rev. 2022, 80, 1856–1895. [Google Scholar] [CrossRef]
- Ha, V.; Bonner, A.J.; Jadoo, J.K.; Beyene, J.; Anand, S.S.; de Souza, R.J. The effects of various diets on glycemic outcomes during pregnancy: A systematic review and network meta-analysis. PLoS ONE 2017, 12, e0182095. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Higgins, J.; Deeks, J. Chapter 5: Collecting Data; Cochrane: 2021. Available online: https://training.cochrane.org/handbook/current/chapter-05 (accessed on 1 November 2022).
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Nikolakopoulou, A.; Higgins, J.P.T.; Papakonstantinou, T.; Chaimani, A.; Del Giovane, C.; Egger, M.; Salanti, G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020, 17, e1003082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papakonstantinou, T.; Nikolakopoulou, A.; Higgins, J.P.T.; Egger, M.; Salanti, G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 2020, 16. [Google Scholar] [CrossRef] [Green Version]
- Salanti, G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: Many names, many benefits, many concerns for the next generation evidence synthesis tool. Res. Synth. Methods 2012, 3, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Chaimani, A.; Caldwell, D.; Li, T.; Higgins, J.; Salanti, G. Chapter 11: Undertaking Network Meta-Analyses; Cochrane: 2021. Available online: https://training.cochrane.org/handbook/current/chapter-11 (accessed on 1 November 2022).
- Mbuagbaw, L.; Rochwerg, B.; Jaeschke, R.; Heels-Andsell, D.; Alhazzani, W.; Thabane, L.; Guyatt, G.H. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst. Rev. 2017, 6, 79. [Google Scholar] [CrossRef] [Green Version]
- Bucher, H.C.; Guyatt, G.H.; Griffith, L.E.; Walter, S.D. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J. Clin. Epidemiol. 1997, 50, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Dias, S.; Welton, N.J.; Caldwell, D.M.; Ades, A.E. Checking consistency in mixed treatment comparison meta-analysis. Stat. Med. 2010, 29, 932–944. [Google Scholar] [CrossRef]
- Higgins, J.P.; Jackson, D.; Barrett, J.K.; Lu, G.; Ades, A.E.; White, I.R. Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 2012, 3, 98–110. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Barrett, J.K.; Rice, S.; White, I.R.; Higgins, J.P. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat. Med. 2014, 33, 3639–3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaimani, A.; Higgins, J.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef] [Green Version]
- Crichton, G.E.; Howe, P.R.C.; Buckley, J.D.; Coates, A.M.; Murphy, K.J.; Bryan, J. Long-term dietary intervention trials: Critical issues and challenges. Trials 2012, 13, 111. [Google Scholar] [CrossRef]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- White, I. Network meta-analysis. Stata J. 2015, 15, 951–985. [Google Scholar] [CrossRef] [Green Version]
- Chaimani, A.; Salanti, G. Visualizing assumptions and results in network meta-analysis: The network graphs package. Stata J. 2015, 15, 905–950. [Google Scholar] [CrossRef] [Green Version]
- Lawson, J.; Willden, C. Mixture Experiments in R Using mixexp. J. Stat. Softw. 2016, 72, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Koemel, N.A.; Senior, A.M.; Dissanayake, H.U.; Ross, J.; McMullan, R.L.; Kong, Y.; Phang, M.; Hyett, J.; Raubenheimer, D.; Gordon, A.; et al. Maternal dietary fatty acid composition and newborn epigenetic aging-a geometric framework approach. Am. J. Clin. Nutr. 2022, 115, 118–127. [Google Scholar] [CrossRef]
- de la O, V.; Zazpe, I.; Martinez, J.A.; Santiago, S.; Carlos, S.; Zulet, M.A.; Ruiz-Canela, M. Scoping review of Paleolithic dietary patterns: A definition proposal. Nutr. Res. Rev. 2020, 1–29. [Google Scholar] [CrossRef]
- Ghaedi, E.; Mohammadi, M.; Mohammadi, H.; Ramezani-Jolfaie, N.; Malekzadeh, J.; Hosseinzadeh, M.; Salehi-Abargouei, A. Effects of a Paleolithic Diet on Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 634–646. [Google Scholar] [CrossRef]
- Cambeses-Franco, C.; González-García, S.; Feijoo, G.; Moreira, M.T. Is the Paleo diet safe for health and the environment? Sci. Total Environ. 2021, 781. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [Green Version]
- Crosby, L.; Davis, B.; Joshi, S.; Jardine, M.; Paul, J.; Neola, M.; Barnard, N.D. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front. Nutr. 2021, 8, 702802. [Google Scholar] [CrossRef]
- Chiavaroli, L.; Viguiliouk, E.; Nishi, S.K.; Blanco Mejia, S.; Rahelić, D.; Kahleová, H.; Salas-Salvadó, J.; Kendall, C.W.; Sievenpiper, J.L. DASH Dietary Pattern and Cardiometabolic Outcomes: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrients 2019, 11, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-González, M.A.; Gea, A.; Ruiz-Canela, M. The Mediterranean Diet and Cardiovascular Health: A Critical Review. Circ. Res. 2019, 124, 779–798. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022. [Google Scholar] [CrossRef]
- Liu, L.; Jin, R.; Hao, J.; Zeng, J.; Yin, D.; Yi, Y.; Zhu, M.; Mandal, A.; Hua, Y.; Ng, C.K.; et al. Consumption of the Fish Oil High-Fat Diet Uncouples Obesity and Mammary Tumor Growth through Induction of Reactive Oxygen Species in Protumor Macrophages. Cancer Res. 2020, 80, 2564–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Efthimiou, O.; White, I.R. The dark side of the force: Multiplicity issues in network meta-analysis and how to address them. Res. Synth. Methods 2020, 11, 105–122. [Google Scholar] [CrossRef] [PubMed]


| Mediterranean | |||||||||
| −0.03 (−0.27, 0.21) 4 | DASH | ||||||||
| −0.25 (−0.71, 0.22) 4 | −0.21 (−0.70, 0.28) 4 | Paleo | |||||||
| −0.11 (−0.30, 0.07) 3 | −0.08 (−0.32, 0.16) 3 | 0.13 (−0.30, 0.56) 4 | DG-based | ||||||
| −0.23 (−0.85, 0.39) 5 | −0.20 (−0.84, 0.45) 5 | 0.02 (−0.74, 0.78) 4 | −0.12 (−0.74, 0.51) 5 | Low GI/GL | |||||
| −0.01 (−0.20, 0.19) 3 | 0.02 (−0.24, 0.28) 5 | 0.24 (−0.24, 0.71) 4 | 0.10 (−0.10, 0.31) 2 | 0.22 (−0.41, 0.85) 5 | Plant-based | ||||
| −0.03 (−0.18, 0.11) 3 | 0.00 (−0.24, 0.24) 3 | 0.21 (−0.26, 0.69) 4 | 0.08 (−0.12, 0.28) 4 | 0.20 (−0.43, 0.82) 5 | −0.02 (−0.22, 0.18) 4 | Low-fat | |||
| −0.37 (−0.68, −0.05) 3 | −0.33 (−0.70, 0.03) 4 | −0.12 (−0.66, 0.42) 4 | −0.25 (−0.59, 0.08) 4 | −0.14 (−0.82, 0.54) 5 | −0.36 (−0.66, −0.05) 2 | −0.33 (−0.66, −0.01) 4 | Low CHO high-fat | ||
| −0.27 (−0.88, 0.34) 5 | −0.24 (−0.87, 0.39) 5 | −0.03 (−0.78, 0.73) 4 | −0.16 (−0.78, 0.46) 5 | −0.04 (−0.25, 0.16) 5 | −0.26 (−0.88, 0.36) 5 | −0.24 (−0.86, 0.38) 5 | 0.09 (−0.58, 0.76) 5 | High GI/GL | |
| −0.29 (−0.41, −0.16) 3 | −0.25 (−0.47, −0.04) 3 | −0.04 (−0.50, 0.42) 4 | −0.17 (−0.33, −0.02) 3 | −0.06 (−0.67, 0.55) 5 | −0.28 (−0.44, −0.12) 3 | −0.26 (−0.41, −0.11) 3 | 0.08 (−0.22, 0.38) 3 | −0.01 (−0.61, 0.58) 5 | Western habitual |
| Inflammation | Glycemic Control | Lipids and Apolipoproteins | All-Outcomes-Combined 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| hsCRP | IL-6 | Combined 2 | Glucose | Insulin | HOMA-IR | Combined 2 | TC | LDL-c | HDL-c | TG | ApoB | ApoA1 | Combined 2 | ||
| Mediterranean | 56.4 | 59.7 | 58.1 | 50.0 | 63.1 | 40.4 | 51.2 | 65.3 | 79.2 | 57.9 | 72.0 | 62.8 | 41.3 | 63.1 | 57.4 |
| DASH | 71.3 | 71.3 | 61.0 | 58.2 | 59.6 | 75.2 | 70.3 | 18.1 | 63.0 | 77.9 | 33.9 | 56.4 | 62.4 | ||
| Paleo | 79.0 | 95.0 | 87.0 | 35.3 | 70.7 | 89.7 | 65.2 | 32.9 | 35.5 | 78.6 | 51.1 | 49.5 | 67.2 | ||
| Dietary guidelines-based | 73.4 | 31.0 | 52.2 | 16.7 | 59.3 | 58.7 | 44.9 | 54.2 | 52.3 | 37.4 | 44.7 | 64.3 | 37.9 | 48.5 | 48.5 |
| Low GI/GL | 48.2 | 31.1 | 31.3 | 36.9 | 51.1 | 42.2 | 43.2 | 29.8 | 41.6 | ||||||
| Plant-based | 44.9 | 44.9 | 57.6 | 27.6 | 90.4 | 58.5 | 76.0 | 75.6 | 16.9 | 27.5 | 49.6 | 21.1 | 44.5 | 49.3 | |
| Low-fat | 14.5 | 20.4 | 17.5 | 48.2 | 78.5 | 35.6 | 54.1 | 74.3 | 70.7 | 28.8 | 47.6 | 77.0 | 45.1 | 57.3 | 42.9 |
| Low CHO high-fat | 31.2 | 31.2 | 80.8 | 51.9 | 66.4 | 14.8 | 16.8 | 92.6 | 91.3 | 4.3 | 93.4 | 52.2 | 49.9 | ||
| Traditional Mexican | 58.8 | 58.8 | |||||||||||||
| High GI/GL | 66.3 | 25.8 | 35.3 | 42.5 | 34.2 | 34.3 | 55.2 | 18.4 | 35.5 | ||||||
| Western habitual | 29.3 | 43.9 | 36.6 | 27.2 | 33.8 | 18.6 | 26.5 | 22.1 | 23.1 | 71.5 | 54.6 | 14.1 | 77.2 | 43.8 | 35.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Mijatovic, J.; Li, A.; Koemel, N.; Nasir, R.; Toniutti, C.; Bell-Anderson, K.; Skilton, M.; O’Leary, F. Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach. Nutrients 2023, 15, 76. https://doi.org/10.3390/nu15010076
Liang S, Mijatovic J, Li A, Koemel N, Nasir R, Toniutti C, Bell-Anderson K, Skilton M, O’Leary F. Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach. Nutrients. 2023; 15(1):76. https://doi.org/10.3390/nu15010076
Chicago/Turabian StyleLiang, Shuang, Jovana Mijatovic, Ang Li, Nicholas Koemel, Reeja Nasir, Clémence Toniutti, Kim Bell-Anderson, Michael Skilton, and Fiona O’Leary. 2023. "Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach" Nutrients 15, no. 1: 76. https://doi.org/10.3390/nu15010076
APA StyleLiang, S., Mijatovic, J., Li, A., Koemel, N., Nasir, R., Toniutti, C., Bell-Anderson, K., Skilton, M., & O’Leary, F. (2023). Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach. Nutrients, 15(1), 76. https://doi.org/10.3390/nu15010076