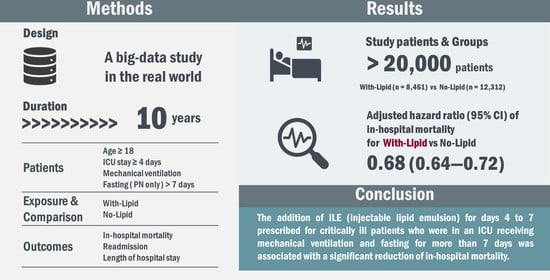
Injectable Lipid Emulsion and Clinical Outcomes in Patients Exclusively Receiving Parenteral Nutrition in an ICU: A Retrospective Cohort Study Using a Japanese Medical Claims Database
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Patient Population
2.3. Patient Group
2.4. Endpoints
2.5. Data Extraction
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Endpoints
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patkova, A.; Joskova, V.; Havel, E.; Kovarik, M.; Kucharova, M.; Zadak, Z.; Hronek, M. Energy, Protein, Carbohydrate, and Lipid Intakes and Their Effects on Morbidity and Mortality in Critically Ill Adult Patients: A Systematic Review. Adv. Nutr. 2017, 8, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Skorepa, P.; Sobotka, O.; Vanek, J.; Ticha, A.; Fortunato, J.; Manak, J.; Blaha, V.; Horacek, J.M.; Sobotka, L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients 2020, 12, 1373. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef] [PubMed]
- Compher, C.; Bingham, A.L.; McCall, M.; Patel, J.; Rice, T.W.; Braunschweig, C.; McKeever, L. Guidelines for the provision of nutrition support therapy in the adult critically ill patient: The American Society for Parenteral and Enteral Nutrition. JPEN J. Parenter. Enter. Nutr. 2022, 46, 12–41. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Horikoshi, Y.; Kamoshita, S.; Kuroda, A.; Moriya, T. Nutritional management of ICU patients receiving mechanical ventilation: A retrospective cohort study using a medical claims database. Clin. Nutr. Open Sci. 2022, 42, 84–98. [Google Scholar] [CrossRef]
- Christensen, M.L.; Ayers, P.; Boullata, J.I.; Guenter, P.; Gura, K.M.; Holcombe, B.; Seres, D.S.; Sacks, G.S.; ASPEN PN Safety Committee. Lipid Injectable Emulsion Survey with Gap Analysis. Nutr. Clin. Pract. 2017, 32, 694–702. [Google Scholar] [CrossRef]
- Heyland, D.K.; MacDonald, S.; Keefe, L.; Drover, J.W. Total parenteral nutrition in the critically ill patient: A meta-analysis. JAMA 1998, 280, 2013–2019. [Google Scholar] [CrossRef]
- Wu, S.C.; Chen, T.A.; Cheng, H.T.; Chang, Y.J.; Wang, Y.C.; Tzeng, C.W.; Hsu, C.H.; Muo, C.H. Lipid-Free PN is Associated with an Increased Risk of Hyperbilirubinemia in Surgical Critically Ill Patients with Admission Hepatic Disorder: A Retrospective Observational Study. Ther. Clin. Risk Manag. 2021, 17, 1001–1010. [Google Scholar] [CrossRef]
- Hastings, J.; Ridley, E.J.; Bianchet, O.; Roodenburg, O.; Levkovich, B.; Scheinkestel, C.; Pilcher, D.; Udy, A. Does Propofol Sedation Contribute to Overall Energy Provision in Mechanically Ventilated Critically Ill Adults? A Retrospective Observational Study. JPEN J. Parenter. Enter. Nutr. 2018, 42, 748–757. [Google Scholar] [CrossRef]
- WHO Europe. Body Mass Index-BMI. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi?source=post_page (accessed on 18 June 2023).
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional evaluation: The Barthel Index. Md State Med. J. 1965, 14, 61–65. [Google Scholar]
- Shigematsu, K.; Nakano, H.; Watanabe, Y. The eye response test alone is sufficient to predict stroke outcome -Reintroduction of Japan Coma Scale: A cohort study. BMJ Open 2013, 3, e002736. [Google Scholar] [CrossRef]
- Krishnan, J.A.; Parce, P.B.; Martinez, A.; Diette, G.B.; Brower, R.G. Caloric intake in medical ICU patients: Consistency of care with guidelines and relationship to clinical outcomes. Chest 2003, 124, 297–305. [Google Scholar] [CrossRef]
- Bendavid, I.; Singer, P.; Theilla, M.; Themessl-Huber, M.; Sulz, I.; Mouhieddine, M.; Schuh, C.; Mora, B.; Hiesmayr, M. NutritionDay ICU: A 7 year worldwide prevalence study of nutrition practice in intensive care. Clin. Nutr. 2017, 36, 1122–1129. [Google Scholar] [CrossRef]
- Ridley, E.J.; Peake, S.L.; Jarvis, M.; Deane, A.M.; Lange, K.; Davies, A.R.; Chapman, M.; Heyland, D. Nutrition Therapy in Australia and New Zealand Intensive Care Units: An International Comparison Study. JPEN J. Parenter. Enter. Nutr. 2018, 42, 1349–1357. [Google Scholar] [CrossRef]
- Yatabe, T.; Egi, M.; Sakaguchi, M.; Ito, T.; Inagaki, N.; Kato, H.; Kaminohara, J.; Konishi, A.; Takahashi, M.; Tatsumi, H.; et al. Influence of Nutritional Management and Rehabilitation on Physical Outcome in Japanese Intensive Care Unit Patients: A Multicenter Observational Study. Ann. Nutr. Metab. 2019, 74, 35–43. [Google Scholar] [CrossRef]
- Tappy, L.; Schwarz, J.M.; Schneiter, P.; Cayeux, C.; Revelly, J.P.; Fagerquist, C.K.; Jequier, E.; Chiolero, R. Effects of isoenergetic glucose-based or lipid-based parenteral nutrition on glucose metabolism, de novo lipogenesis, and respiratory gas exchanges in critically ill patients. Crit. Care Med. 1998, 26, 860–867. [Google Scholar] [CrossRef]
- Al-Yousif, N.; Rawal, S.; Jurczak, M.; Mahmud, H.; Shah, F.A. Endogenous Glucose Production in Critical Illness. Nutr. Clin. Pract. 2021, 36, 344–359. [Google Scholar] [CrossRef]
- Nordenström, J.; Carpentier, Y.A.; Askanazi, J.; Robin, A.P.; Elwyn, D.H.; Hensle, T.W.; Kinney, J.M. Metabolic utilization of intravenous fat emulsion during total parenteral nutrition. Ann. Surg. 1982, 196, 221–231. [Google Scholar] [CrossRef]
- Yamana, H.; Moriwaki, M.; Horiguchi, H.; Kodan, M.; Fushimi, K.; Yasunaga, H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J. Epidemiol. 2017, 27, 476–482. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; De Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study; working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Categories | Patient Groups | |
|---|---|---|---|
| No-Lipid n = 12,312 | With-Lipid n = 8461 | ||
| Age, years, n (%) | <60 | 1924 (15.6) | 1713 (20.2) |
| 60–69 | 2286 (18.6) | 1763 (20.8) | |
| 70–79 | 3577 (29.1) | 2676 (31.6) | |
| 80–89 | 3712 (30.1) | 2030 (24.0) | |
| ≥90 | 813 (6.6) | 279 (3.3) | |
| Sex, n (%) | Male | 7474 (60.7) | 5678 (67.1) |
| Female | 4838 (39.3) | 2783 (32.9) | |
| Body mass index, kg/m2, n (%) | <16 | 880 (7.1) | 439 (5.2) |
| 16–18.5 | 1866 (15.2) | 1027 (12.1) | |
| 18.5–22.5 | 4523 (36.7) | 2955 (34.9) | |
| 22.5–25 | 2386 (19.4) | 1895 (22.4) | |
| ≥25 | 2657 (21.6) | 2145 (25.4) | |
| Beds in admission hospital, n (%) | <200 | 323 (2.6) | 362 (4.3) |
| ≥200, <500 | 7135 (58.0) | 4741 (56.0) | |
| ≥500 | 4854 (39.4) | 3358 (39.7) | |
| Admission year, n (%) | 2010–2011 | 409 (3.3) | 299 (3.5) |
| 2012–2013 | 1266 (10.3) | 850 (10.0) | |
| 2014–2015 | 2539 (20.6) | 1770 (20.9) | |
| 2016–2017 | 3554 (28.9) | 2485 (29.4) | |
| 2018–2019 | 3923 (31.9) | 2642 (31.2) | |
| 2020 | 621 (5.0) | 415 (4.9) | |
| Primary diagnosis 2, n (%) | Sepsis | 681 (5.5) | 570 (6.7) |
| Neoplasm | 1208 (9.8) | 1064 (12.6) | |
| Diseases of the nervous system | 555 (4.5) | 215 (2.5) | |
| Ischemic heart disease | 658 (5.3) | 693 (8.2) | |
| Heart failure | 800 (6.5) | 340 (4.0) | |
| Cerebrovascular disorders | 1664 (13.5) | 747 (8.8) | |
| Other circulatory system diseases | 1664 (13.5) | 1603 (18.9) | |
| Pneumonia | 549 (4.5) | 257 (3.0) | |
| Interstitial respiratory diseases | 365 (3.0) | 233 (2.8) | |
| Other respiratory diseases | 926 (7.5) | 383 (4.5) | |
| Diseases of the digestive system | 1777 (14.4) | 1324 (15.6) | |
| Kidney diseases | 162 (1.3) | 109 (1.3) | |
| Injury, poisoning, other consequences external causes | 551 (5.4) | 395 (4.7) | |
| Other | 752 (6.1) | 528 (6.2) | |
| Charlson Comorbidity Index, n (%) | 0 | 6687 (54.3) | 4298 (50.8) |
| 1–2 | 4072 (33.1) | 2962 (35.0) | |
| ≥3 | 1553 (12.6) | 1201 (14.2) | |
| Barthel Index, n (%) | 100 | 2092 (17.0) | 2131 (25.2) |
| 65–95 | 376 (3.1) | 312 (3.7) | |
| 45–60 | 381 (3.1) | 268 (3.2) | |
| 25–40 | 266 (2.2) | 149 (1.8) | |
| 5–20 | 564 (4.6) | 342 (4.0) | |
| 0 | 6840 (55.6) | 4042 (47.8) | |
| NA | 1793 (14.6) | 1217 (14.4) | |
| Japan Coma Scale, n (%) | 0 | 5350 (43.5) | 4680 (55.3) |
| 1–3 | 2123 (17.2) | 1418 (16.8) | |
| 10–30 | 1188 (9.6) | 710 (8.4) | |
| 100–300 | 3650 (29.6) | 1653 (19.5) | |
| NA | 1 (0.0) | 0 (0.0) | |
| Surgery 3, n (%) | Cardiovascular | 765 (6.2) | 1213 (14.3) |
| Gastroenterological | 2383 (19.4) | 2109 (24.9) | |
| Cerebrovascular | 923 (7.5) | 437 (5.2) | |
| Respiratory | 29 (0.2) | 41 (0.5) | |
| Orthopedic | 32 (0.3) | 40 (0.5) | |
| Urological/Gynecological | 63 (0.5) | 41 (0.5) | |
| Multiple surgeries | 41 (0.3) | 62 (0.7) | |
| Other | 172 (1.4) | 194 (2.3) | |
| No surgery | 7904 (64.2) | 4324 (51.1) | |
| Prescription/treatment 4, n (%) | Catecholamines | 8530 (69.3) | 6519 (77.0) |
| Transfusion | 5257 (42.7) | 5129 (60.6) | |
| Albumin | 4961 (40.3) | 5032 (59.5) | |
| Renal replacement therapy | 2319 (18.8) | 2225 (26.3) | |
| Intra-aortic balloon pump | 746 (6.1) | 920 (10.9) | |
| Plasmapheresis | 62 (0.5) | 44 (0.5) | |
| ECMO | 367 (3.0) | 531 (6.3) | |
| Nutritional support team | 233 (1.9) | 173 (2.0) | |
| Rehabilitation 5 | 5075 (41.2) | 3838 (45.4) | |
| Parenteral nutrition 6, median (Q1, Q3) | Energy, kcal/kg/d | 6.9 (3.8, 11.7) | 10.6 (6.8, 15.7) |
| Amino acids, g/kg/d | 0.16 (0.00, 0.38) | 0.23 (0.04, 0.43) | |
| Lipid, g/kg/d | 0.00 (0.00, 0.01) | 0.13 (0.07, 0.22) | |
| Carbohydrate, g/kg/d | 1.5 (0.9, 2.5) | 2.0 (1.3, 3.1) | |
| Days without nutrition from day 8 7, median (Q1, Q3) | 0.0 (0.0, 1.0) | 0.0 (0.0, 0.0) | |
| Patient Group | Odds Ratio/Regression Coefficient (95% CI) 2 | ||||
|---|---|---|---|---|---|
| No-Lipid n = 12,312 | With-Lipid n = 8461 | Unadjusted | Model 1 3 | Model 2 4 | |
| In-hospital mortality, n (%) | 5863 (47.6) | 2958 (35.0) | 0.59 (0.56–0.63) | 0.62 (0.58–0.67) | 0.66 (0.62–0.71) |
| Hospital readmission 5,6, n (%) | 270 (4.2) | 241 (4.4) | 1.03 (0.85–1.25) | 0.96 (0.78–1.18) | 0.94 (0.76–1.15) |
| Hospital LOS 5, median (Q1, Q3) | 45 (29, 71) | 48 (31, 73) | 1.96 (−0.43–4.35) | 0.58 (−1.88–3.04) | 0.78 (−1.69–3.25) |
| Patient Subgroups | |||
|---|---|---|---|
| ILE-only | ILE+ propofol | Propofol-only | |
| n = 1073 | n = 541 | n = 6847 | |
| Unadjusted odds ratio/regression coefficient (95% CI) 2 | |||
| In-hospital mortality | 0.74 (0.64–0.85) | 0.63 (0.52–0.77) | 0.57 (0.54–0.61) |
| Hospital readmission 3,4 | 1.08 (0.71–1.65) | 1.01 (0.56–1.82) | 1.02 (0.84–1.26) |
| Hospital LOS 5, median | 2.34 (−3.02–7.70) | 5.24 (−2.08–12.55) | 1.67 (−0.86–4.19) |
| Model 1 5 odds ratio/regression coefficient (95% CI) 2 | |||
| In-hospital mortality | 0.82 (0.71–0.96) | 0.68 (0.55–0.84) | 0.59 (0.55–0.64) |
| Hospital readmission 3,4 | 1.01 (0.65–1.56) | 0.93 (0.50–1.71) | 0.96 (0.77–1.19) |
| Hospital LOS 3 | 2.39 (−2.91–7.70) | 2.97 (−4.27–10.21) | 0.10 (−2.52–2.71) |
| Model 2 6 odds ratio/regression coefficient (95% CI) 2 | |||
| In-hospital mortality | 0.87 (0.75–1.01) | 0.71 (0.57–0.87) | 0.63 (0.58–0.68) |
| Hospital readmission 3,4 | 0.98 (0.64–1.52) | 0.92 (0.50–1.70) | 0.93 (0.75–1.16) |
| Hospital LOS 3 | 2.65 (−2.67–7.96) | 3.18 (−4.07–10.42) | 0.29 (−2.33–2.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasuda, H.; Horikoshi, Y.; Kamoshita, S.; Kuroda, A.; Moriya, T. Injectable Lipid Emulsion and Clinical Outcomes in Patients Exclusively Receiving Parenteral Nutrition in an ICU: A Retrospective Cohort Study Using a Japanese Medical Claims Database. Nutrients 2023, 15, 2797. https://doi.org/10.3390/nu15122797
Yasuda H, Horikoshi Y, Kamoshita S, Kuroda A, Moriya T. Injectable Lipid Emulsion and Clinical Outcomes in Patients Exclusively Receiving Parenteral Nutrition in an ICU: A Retrospective Cohort Study Using a Japanese Medical Claims Database. Nutrients. 2023; 15(12):2797. https://doi.org/10.3390/nu15122797
Chicago/Turabian StyleYasuda, Hideto, Yuri Horikoshi, Satoru Kamoshita, Akiyoshi Kuroda, and Takashi Moriya. 2023. "Injectable Lipid Emulsion and Clinical Outcomes in Patients Exclusively Receiving Parenteral Nutrition in an ICU: A Retrospective Cohort Study Using a Japanese Medical Claims Database" Nutrients 15, no. 12: 2797. https://doi.org/10.3390/nu15122797
APA StyleYasuda, H., Horikoshi, Y., Kamoshita, S., Kuroda, A., & Moriya, T. (2023). Injectable Lipid Emulsion and Clinical Outcomes in Patients Exclusively Receiving Parenteral Nutrition in an ICU: A Retrospective Cohort Study Using a Japanese Medical Claims Database. Nutrients, 15(12), 2797. https://doi.org/10.3390/nu15122797