A Comprehensive Review of the Effect of Honey on Human Health
Highlights
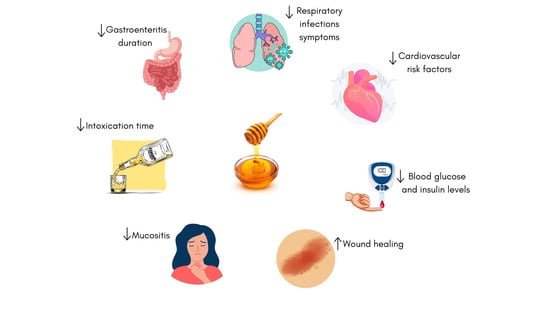
- Honey from sources like clover, thyme, buckwheat, and manuka has shown positive effects on health.
- Honey improves metabolic and cardiovascular health by enhancing insulin sensitivity, reducing blood glucose, improving the lipid profile, and reducing oxidative stress with the help of their bioactive compounds.
- Honey promotes intestinal health and wound healing and helps manage oral mucositis in cancer patients, as well as the symptoms in children with upper respiratory infections (URTIs).
- More robust studies, taking into account honey types, dosages, and trial methods, are needed to establish clear guidelines for the therapeutic use of honey.
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Cardiovascular and Metabolic Risk Factors
3.1.1. Healthy Subjects
3.1.2. Overweight or Obese Subjects
3.1.3. Diabetic Subjects
3.1.4. Subjects with Hyperlipidemia
3.2. Glucose Tolerance
3.2.1. Healthy Subjects
3.2.2. Diabetic Subjects
| Honey | Dose | Test Duration | Subjects | Physiological Parameter | Effect | References |
|---|---|---|---|---|---|---|
| Basswood (linden) honey | 75 g vs. glucose-fructose | 120 min | Healthy men 27.7 years | Increase in BGL | ↓ | [23] |
| AUC for glucose | ↓ a | |||||
| Increase in BIL | ↓ a | |||||
| Increase in C-peptide | ↓ | |||||
| Natural honey | 75 g vs. dextrose | 180 min | Healthy subjects 25–42 years | Increase in BGL | ↓ | [21] |
| Increase in BIL | ↓ a | |||||
| Increase in C-peptide | ↓ a | |||||
| 70 g vs. glucose | Type II diabetic patients | Increase in BGL | ↓ a | |||
| Sue Bee honey (clover honey) 100% pure | 75 g honey vs. glucose | 120 min | Type II diabetic patients 50 ± 9.7 years | Increase in BGL | ↓ a | [24] |
| Unprocessed Egyptian clover honey supplied by a beekeeper | 2.3 g/kg | 120 min | Healthy subjects and type I diabetic patients 10.02 years | Increase in BGL | ↓ a | [25] |
| Increase in C-peptide | ↑ a |
3.3. Appetite and Food Intake
3.3.1. Healthy Subjects
3.3.2. Diabetic Subjects
3.4. Alcohol Metabolism
3.5. Cancer
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | Reference |
|---|---|---|---|---|---|---|
| Natural Baran-Baghro honey from Iran | 1:20 honey:water Mouthwash | 4 w | Acute myeloid leukemia patients receiving chemotherapy >18 years | Mucositis severity | ↓ *,a | [29] |
| Body weight | ↑ *,a | |||||
| Pure and filtered thyme honey | 1:5 honey:water Mouthwash | 6 m | Head and neck cancer patients receiving radiotherapy 61.53 years | Mucositis severity | ↓ *,a | [30] |
| Weight loss | ↓ a | |||||
| Global health | ↑ *,a | |||||
| Life quality | ↑ *,a | |||||
| Western Ghats forests honey | 20 mL Mouthwash | 6 w | Oral carcinoma patients receiving radiotherapy >18 years | Mucositis severity | ↓ a | [31] |
| Pure and filtered natural clover honey | 20 mL pure honey Rinse + swallow | 7 w | Head and neck cancer patients receiving chemotherapy 48.20 ± 15.63 years | Mucositis severity | ↓ a | [32] |
| Candida colonization | ↓ a | |||||
| Pure natural honey from Thymus and Astragale in the Albroz mountains in northern Iran | 20 mL pure honey Rinse + swallow | 6 w | Head and neck cancer patients receiving radiotherapy 57.0 ± 12.0 years | Mucositis severity | ↓ *,a | [33] |
| Weight loss | ↓ a | |||||
| Tea plant honey from Cameron Highland of peninsular Malaysia | 20 mL pure honey Rinse + swallow | 7 w | Head and neck cancer patients receiving radiotherapy 14–89 years | Mucositis severity | ↓ a | [34] |
| Body weight | ↑ a | |||||
| Pure and filtered thyme honey | 1:5 honey:water Rinse + swallow | 6 w | Head and neck cancer patients receiving radiotherapy or chemotherapy or surgery 61.53 ± 13.50 years | Xerostomia level | ↓ a | [35] |
| Quality life | ↑ a | |||||
| Pain | ↓ a | |||||
| Dysphagia | ↓ a | |||||
| Irradiated organic manuka honey | 5 mL Rinse + swallow | 6 w | Head and neck cancer patients receiving radiotherapy | Mucositis severity | ↓ | [36] |
| Active manuka honey | 20 mL (98% honey) Rinse + swallow | 6 w | Head and neck cancer patients receiving radiotherapy 38–85 years | Incidence of servere mucositis | ↑ | [37] |
| Mucositis severity | ↓ | |||||
| Mucositis duration | ↓ | |||||
| Egyptian clover honey from El Mahala, Gharbia Governorate | 0.5 g/kg/d Rinse + swallow | 10 d | Lymphoblastic leukaemia patients receiving chemotherapy 6.9 ± 3.8 years | Mucositis recovery time | ↓ a | [38] |
| Turkish Flower honey from the highlands of Zonguldak Province, in the Western Black Sea Region of Turkey | 3.70–30.96 g Rinse + swallow | 21 d | Children treated in a paediatric intensive care unit (PICU) 7.25 years | Mucositis severity | ↓ *,a | [39] |
| Life-Mel honey from Express Honey, Tzuf Globus, Israel | 5 g/d | 5 d | Cancer patients with neutropenia 57 years | Neutrophil level | ↑ * | [40] |
| Haemoglobin level | ↑ | |||||
| Thrombocytes level | ↑ | |||||
| Egyptian unprocessed clover honey collected from Al Mahala-Gharbia Governorate | 2.5 g/kg twice weekly | Crossover Two 12 w periods | Children with acute lymphoblastic leukemia 5.4 ± 2.4 years | Febrile neutropenia episodes | ↓ a | [41] |
| Number of patients admitted in hospital | ↓ | |||||
| Duration of hospital stay | ↓ | |||||
| Haemoglobin level | ↑ a |
3.6. Cough and Gastroenteritis in Infants
3.7. Antimicrobial and Wound Healing Effects
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | References |
|---|---|---|---|---|---|---|
| Buckwheat honey | Children aged 2 to 5 (1/2 teaspoon), 6 to 11 (1 teaspoon), 12 to 18 (2 teaspoons) Single dose | 1 d | Children with upper URTIs 5.02 ± 3.99 years | Cough frequency | ↓ a | [42] |
| Combined symptom score | ↓ a | |||||
| Bothersome cough | ↓ | |||||
| Cough severity | ↓ | |||||
| Sleep quality | ↑ | |||||
| Parents’ sleep quality | ↑ | |||||
| Iranian natural honey from Kafi-Abad, Yazd | 2.5 mL Single dose | 1 d | Children with URTIs 3.15 ± 0.93 years | Cough frequency | ↓ a | [43] |
| Cough severity | ↓a | |||||
| Sleep quality | ↑ a | |||||
| Parents’ sleep quality | ↑ a | |||||
| Eucalyptus, citrus or Labiatae honey | 10 g Single dose | 1 d | Children with URTIs 2.4 years | Cough frequency | ↓ a | [44] |
| Combined symptom score | ↓ a | |||||
| Bothersome cough | ↓ a | |||||
| Cough severity | ↓ a | |||||
| Sleep quality | ↑ a | |||||
| Parents’ sleep quality | ↑ a | |||||
| Nairobi dark honey | Children aged 1 to 2 (2.5 mL), 2 to 6 (5 mL), 6 to 12 (7.5 mL) Three times daily | 5 d | Children with a common cold 1–12 years | Cough frequency | ↓ a | [45] |
| Combined symptom score | ↓ a | |||||
| Bothersome cough | ↓ a | |||||
| Cough severity | ↓ a | |||||
| Cough duration | ↓ a | |||||
| Sleep quality | ↑ a | |||||
| Parents’ sleep quality | ↑ a | |||||
| Two kinds of Iranian honey: Kimia honey and Golha honey | Children aged 1 to 6 (2.5 mL), 7 to 12 (5 mL), Two doses | 2 d | Children with URTIs 3.5 ± 1.6 years | Cough frequency | ↓ b | [46] |
| Combined standard score | ↓ a | |||||
| Bothersome cough | ↓ a | |||||
| Sleep quality | ↑ b | |||||
| Parents’ sleep quality | ↑ a | |||||
| Acacia honey | 3 mL Single dose | 2 d | Children with URTIs 2.5 years | Cough frequency | ↓ | [47] |
| Combined symptom score | ↓ | |||||
| Bothersome cough | ↓ | |||||
| Cough severity | ↓ | |||||
| Cough duration | ↑ | |||||
| Sleep quality | ↑ | |||||
| Pure honey | 50 mL/L of rehydration solution vs. 50 mL/L of glucose | Duration of gastroenteritis | Children with gastroenteritis 1.39 ± 1.82 years | Bacterial gastroenteritis recovery time | ↓ a | [48] |
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | References |
|---|---|---|---|---|---|---|
| Multifloral processed honey | 1:1 honey:water Mouthrinse 10 mL twice a day | 5 d | Healthy subjects 20–24 years | Tooth plaque | ↓ * | [49] |
| Iranian honey from Chaharmahal and Bakhtiari region | 70:30 honey:neutral cream 5 g/d | 7 d | Women with vulvovaginal candidiasis 34.3 ± 8.6 years | Inflammation | ↓ * | [50] |
| Discharge | ↓ * | |||||
| Itching | ↓ * | |||||
| Natural raw honey | Honey-soaked gauze vs. medical solution | 21 d | Children with pyomyositis abcesses 4.5 ± 4.0 years | Wound healing | ↑ a | [51] |
| Duration of hospital stay | ↓ a | |||||
| Iranian honey from Qamsar region | 30% honey cream 1 knuckle/d | 14 d | Nulliparous women with episiotomy wound 24.7 ± 4.0 years | Discharge | ↓ a | [52] |
| Wound healing | ↑ a | |||||
| Pain | ↓ | |||||
| Beri-irradiated honey collected from Karak, Pakistan | Once or twice daily or every 48 h | 4 m | Patients with diabetic foot ulcers 54 years | Wound healing | ↑ a | [53] |
| Wound healing time | ↓ a | |||||
| Pure raw untreated clover honey supplied by the Firm of Faculty of Agriculture, Alexandria University | Honey-soaked gauze | 3 m | Patients with diabetic foot ulcers 52.3 years | Ulcer size | ↓ * | [54] |
| Ulcer grade | ↓ * | |||||
| Ulcer stage | ↓ * | |||||
| Inflammation | ↓ * | |||||
| Discharge | ↓ * | |||||
| Wound healing | ↑ * | |||||
| Clean non-sterile pure honey packed by Barnes for Honey Cooperation of Australia | Once daily | 36 d | Patients with diabetic foot ulcers 31–51 years | Wound healing time | ↓ | [55] |
| Discharge | ↓ | |||||
| Edema | ↓ |
3.8. Other Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Insights, F.B. Market Value of Honey Worldwide from 2019 to 2028 (in Billion U.S. Dollars). Available online: https://www.statista.com/statistics/933928/global-market-value-of-honey/ (accessed on 9 December 2022).
- Jha, A. Natural Honey: At the Tip of the Beehive. Available online: https://www.tpci.in/indiabusinesstrade/blogs/natural-honey-at-the-tip-of-the-beehive/ (accessed on 7 December 2022).
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent Advances in the Analysis of Phenolic Compounds in Unifloral Honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baloš, M.M.Ž.; Popov, N.S.; Radulović, J.Z.P.; Stojanov, I.M.; Jakšić, S.M. Sugar profile of different floral origin honeys from Serbia. J. Apic. Res. 2020, 59, 398–405. [Google Scholar] [CrossRef]
- Jasicka-Misiak, I.; Makowicz, E.; Stanek, N. Chromatographic fingerprint, antioxidant activity, and colour characteristic of polish goldenrod (Solidago virgaurea L.) honey and flower. Eur. Food Res. Technol. 2018, 244, 1169–1184. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P.; et al. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Olivoto, T.; Nardino, M.; Carvalho, I.R.; Follmann, D.N.; Szareski, V.I.; Ferrari, M.; de Pelegrin, A.J.; de Souza, V.Q. Plant secondary metabolites and its dynamical systems of induction in response to environmental factors: A review. Afr. J. Agric. Res. 2017, 12, 71–84. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.R.; Ukaegbu, C.I.; Hamid, H.A.; Alara, O.R. Evaluation of antioxidant and antibacterial activities of the stems of Flammulina velutipes and Hypsizygus tessellatus (white and brown var.) extracted with different solvents. J. Food Meas. Charact. 2018, 12, 1947–1961. [Google Scholar] [CrossRef]
- Klepacka, J.; Gujska, E.; Michalak, J. Phenolic Compounds as Cultivar- and Variety-distinguishing Factors in Some Plant Products. Plant Foods Hum. Nutr. 2011, 66, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Zawawi, N.; Chong, P.J.; Mohd Tom, N.N.; Saiful Anuar, N.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing Relationship between Vitamins, Total Phenolic and Total Flavonoid Content and Antioxidant Activities in Various Honey Types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef]
- Rasad, H.; Entezari, M.H.; Ghadiri, E.; Mahaki, B.; Pahlavani, N. The effect of honey consumption compared with sucrose on lipid profile in young healthy subjects (randomized clinical trial). Clin. Nutr. ESPEN 2018, 26, 8–12. [Google Scholar] [CrossRef]
- Majid, M.; Younis, M.A.; Naveed, A.K.; Shah, M.U.; Azeem, Z.; Tirmizi, S.H. Effects of natural honey on blood glucose and lipid profile in young healthy Pakistani males. J. Ayub Med. Coll. Abbottabad 2013, 25, 42–45. [Google Scholar]
- Al-Tamimi, A.M.B.; Petrisko, M.; Hong, M.Y.; Rezende, L.; Clayton, Z.S.; Kern, M. Honey does not adversely impact blood lipids of adult men and women: A randomized cross-over trial. Nutr. Res. 2020, 74, 87–95. [Google Scholar] [CrossRef]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.R.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.R.; Aghasizadeh, R.; et al. Natural Honey and Cardiovascular Risk Factors; Effects on Blood Glucose, Cholesterol, Triacylglycerole, CRP, and Body Weight Compared with Sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef] [Green Version]
- Farakla, I.; Koui, E.; Arditi, J.; Papageorgiou, I.; Bartzeliotou, A.; Papadopoulos, G.E.; Mantzou, A.; Papathanasiou, C.; Dracopoulou, M.; Papastamataki, M.; et al. Effect of honey on glucose and insulin concentrations in obese girls. Eur. J. Clin. Investig. 2018, 49, e13042. [Google Scholar] [CrossRef]
- Raatz, S.K.; Johnson, L.K.; Picklo, M.J. Consumption of Honey, Sucrose, and High-Fructose Corn Syrup Produces Similar Metabolic Effects in Glucose-Tolerant and -Intolerant Individuals. J. Nutr. 2015, 145, 2265–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ab Wahab, S.Z.; Nik Hussain, N.H.; Zakaria, R.; Abdul Kadir, A.; Mohamed, N.; Tohit, N.M.; Norhayati, M.N.; Hassan, I.I. Long-term effects of honey on cardiovascular parameters and anthropometric measurements of postmenopausal women. Complement. Ther. Med. 2018, 41, 154–160. [Google Scholar] [CrossRef]
- Bahrami, M.; Ataie-Jafari, A.; Hosseini, S.; Foruzanfar, M.H.; Rahmani, M.; Pajouhi, M. Effects of natural honey consumption in diabetic patients: An 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009, 60, 618–626. [Google Scholar] [CrossRef]
- Abdulrhman, M.M.; El-Hefnawy, M.H.; Aly, R.H.; Shatla, R.H.; Mamdouh, R.M.; Mahmoud, D.M.; Mohamed, W.S.; Whitfield, P.; Parry-Strong, A.; Walsh, E.; et al. Metabolic Effects of Honey in Type 1 Diabetes Mellitus: A Randomized Crossover Pilot Study. J. Med. Food 2013, 16, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S.; Whitfield, P.; Parry-Strong, A.; Walsh, E.; Weatherall, M.; Krebs, J.D.; Nwobodo, N.; Akpan, J.; Okorie, U.; Ezeonu, C.; et al. Natural Honey Lowers Plasma Glucose, C-Reactive Protein, Homocysteine, and Blood Lipids in Healthy, Diabetic, and Hyperlipidemic Subjects: Comparison with Dextrose and Sucrose. J. Med. Food 2004, 7, 100–107. [Google Scholar] [CrossRef]
- Münstedt, K.; Hoffmann, S.; Hauenschild, A.; Bülte, M.; von Georgi, R.; Hackethal, A. Effect of Honey on Serum Cholesterol and Lipid Values. J. Med. Food 2009, 12, 624–628. [Google Scholar] [CrossRef]
- Münstedt, K.; Sheybani, B.; Hauenschild, A.; Brüggmann, D.; Bretzel, R.G.; Winter, D.; Soylu, M.; Atayoğlu, T.; İnanç, N.; Silici, S.; et al. Effects of Basswood Honey, Honey-Comparable Glucose-Fructose Solution, and Oral Glucose Tolerance Test Solution on Serum Insulin, Glucose, and C-Peptide Concentrations in Healthy Subjects. J. Med. Food 2008, 11, 424–428. [Google Scholar] [CrossRef]
- Nazir, L.; Samad, F.; Haroon, W.; Kidwai, S.; Siddiqi, S.; Zehravi, M. Comparison of glycaemic response to honey and glucose in type 2 diabetes. J. Pak. Med. Assoc. 2014, 64, 69–71. [Google Scholar] [PubMed]
- Abdulrhman, M.; El Hefnawy, M.; Ali, R.; Hamid, I.A.; El-Goud, A.A.; Refai, D. Effects of honey, sucrose and glucose on blood glucose and C-peptide in patients with type 1 diabetes mellitus. Complement. Ther. Clin. Pract. 2013, 19, 15–19. [Google Scholar] [CrossRef]
- Larson-Meyer, D.E.; Willis, K.S.; Willis, L.M.; Austin, K.J.; Hart, A.M.; Breton, A.B.; Alexander, B.M. Effect of Honey versus Sucrose on Appetite, Appetite-Regulating Hormones, and Postmeal Thermogenesis. J. Am. Coll. Nutr. 2010, 29, 482–493. [Google Scholar] [CrossRef]
- Onyesom, I. Effect of Nigerian citrus (Citrus sinensis Osbeck) honey on ethanol metabolism: Original article. South Afr. Med. J. 2004, 94, 984–986. [Google Scholar]
- Onyesom, I. Honey-Induced Stimulation of Blood Ethanol Elimination and Its Influence on Serum Triacylglycerol and Blood Pressure in Man. Ann. Nutr. Metab. 2005, 49, 319–324. [Google Scholar] [CrossRef]
- Pour-Fard-Pachekenari, A.K.; Rahmani, A.; Ghahramanian, A.; Jafarabadi, M.A.; Onyeka, T.C.; Davoodi, A. The effect of an oral care protocol and honey mouthwash on mucositis in acute myeloid leukemia patients undergoing chemotherapy: A single-blind clinical trial. Clin. Oral Investig. 2019, 23, 1811–1821. [Google Scholar] [CrossRef]
- Charalambous, M.; Raftopoulos, V.; Paikousis, L.; Katodritis, N.; Lambrinou, E.; Vomvas, D.; Georgiou, M.; Charalambous, A. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur. J. Oncol. Nurs. 2018, 34, 89–97. [Google Scholar] [CrossRef]
- Khanal, B.; Baliga, M.; Uppal, N. Effect of topical honey on limitation of radiation-induced oral mucositis: An intervention study. Int. J. Oral Maxillofac. Surg. 2010, 39, 1181–1185. [Google Scholar] [CrossRef]
- Rashad, U.M.; Al-Gezawy, S.M.; El-Gezawy, E.; Azzaz, A.N. Honey as topical prophylaxis against radiochemotherapy-induced mucositis in head and neck cancer. J. Laryngol. Otol. 2009, 123, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Motallebnejad, M.; Akram, S.; Moghadamnia, A.A.; Moulana, Z.; Omidi, S. The Effect of Topical Application of Pure Honey on Radiation-induced Mucositis: A Randomized Clinical Trial. J. Contemp. Dent. Pract. 2008, 9, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Biswal, B.M.; Zakaria, A.; Ahmad, N.M. Topical application of honey in the management of radiation mucositis. A Preliminary study. Support. Care Cancer 2003, 11, 242–248. [Google Scholar] [CrossRef]
- Charalambous, A.; Lambrinou, E.; Katodritis, N.; Vomvas, D.; Raftopoulos, V.; Georgiou, M.; Paikousis, L.; Charalambous, M. The effectiveness of thyme honey for the management of treatment-induced xerostomia in head and neck cancer patients: A feasibility randomized control trial. Eur. J. Oncol. Nurs. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Hawley, P.; Hovan, A.; McGahan, C.E.; Saunders, D. A randomized placebo-controlled trial of manuka honey for radiation-induced oral mucositis. Support. Care Cancer 2014, 22, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Bardy, J.; Molassiotis, A.; Ryder, W.D.; Mais, K.; Sykes, A.; Yap, B.; Lee, L.; Kaczmarski, E.; Slevin, N. A double-blind, placebo-controlled, randomised trial of active manuka honey and standard oral care for radiation-induced oral mucositis. Br. J. Oral Maxillofac. Surg. 2012, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Abdulrhman, M.; Elbarbary, N.S.; Amin, D.A.; Ebrahim, R.S. Honey and a Mixture of Honey, Beeswax, and Olive Oil–Propolis Extract in Treatment of Chemotherapy-Induced Oral Mucositis: A Randomized Controlled Pilot Study. Pediatr. Hematol. Oncol. 2012, 29, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Sener, D.K.; Aydin, M.; Cangur, S.; Guven, E. The Effect of Oral Care with Chlorhexidine, Vitamin E and Honey on Mucositis in Pediatric Intensive Care Patients: A Randomized Controlled Trial. J. Pediatr. Nurs. 2019, 45, e95–e101. [Google Scholar] [CrossRef] [PubMed]
- Zidan, J.; Shetver, L.; Gershuny, A.; Abzah, A.; Tamam, S.; Stein, M.; Friedman, E. Prevention of Chemotherapy-Induced Neutropenia by Special Honey Intake. Med. Oncol. 2006, 23, 549–552. [Google Scholar] [CrossRef]
- Abdulrhman, M.A.; Hamed, A.A.; Mohamed, S.A.; Hassanen, N.A.A. Effect of honey on febrile neutropenia in children with acute lymphoblastic leukemia: A randomized crossover open-labeled study. Complement. Ther. Med. 2016, 25, 98–103. [Google Scholar] [CrossRef]
- Paul, I.M.; Beiler, J.; McMonagle, A.; Shaffer, M.L.; Duda, L.; Berlin, C.M., Jr. Effect of Honey, Dextromethorphan, and No Treatment on Nocturnal Cough and Sleep Quality for Coughing Children and Their Parents. Arch. Pediatr. Adolesc. Med. 2007, 161, 1140–1146. [Google Scholar] [CrossRef]
- Shadkam, M.N.; Mozaffari-Khosravi, H.; Mozayan, M.R. A Comparison of the Effect of Honey, Dextromethorphan, and Diphenhydramine on Nightly Cough and Sleep Quality in Children and Their Parents. J. Altern. Complement. Med. 2010, 16, 787–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, H.A.; Rozen, J.; Kristal, H.; Laks, Y.; Berkovitch, M.; Uziel, Y.; Kozer, E.; Pomeranz, A.; Efrat, H. Effect of Honey on Nocturnal Cough and Sleep Quality: A Double-blind, Randomized, Placebo-Controlled Study. Pediatrics 2012, 130, 465–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waris, A.; Macharia, W.M.; Njeru, E.K.; Essajee, F. Randomised double blind study to compare effectiveness of honey, salbutamol and placebo in treatment of cough in children with common cold. East Afr. Med. J. 2014, 91, 50–56. [Google Scholar] [PubMed]
- Ayazi, P.; Mahyar, A.; Yousef-Zanjani, M.; Allami, A.; Esmailzadehha, N.; Beyhaghi, T. Comparison of the Effect of Two Kinds of Iranian Honey and Diphenhydramine on Nocturnal Cough and the Sleep Quality in Coughing Children and Their Parents. PLoS ONE 2017, 12, e0170277. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Muta, H.; Hosaka, T.; Ueda, M.; Kishida, K.; Honey and Coughs Study Group of the Society of Ambulatory and General Paediatrics of Japan. Multicentre, randomised study found that honey had no pharmacological effect on nocturnal coughs and sleep quality at 1–5 years of age. Acta Paediatr. 2022, 111, 2157–2164. [Google Scholar] [CrossRef]
- Haffejee, I.E.; Moosa, A. Honey in the treatment of infantile gastroenteritis. Br. Med. J. 1985, 290, 1866–1867. [Google Scholar] [CrossRef] [Green Version]
- Aparna, S.; Srirangarajan, S.; Malgi, V.; Setlur, K.P.; Shashidhar, R.; Setty, S.; Thakur, S. A Comparative Evaluation of the Antibacterial Efficacy of Honey In Vitro and Antiplaque Efficacy in a 4-Day Plaque Regrowth Model In Vivo: Preliminary Results. J. Periodontol. 2012, 83, 1116–1121. [Google Scholar] [CrossRef]
- Banaeian, S.; Sereshti, M.; Rafieian, M.; Farahbod, F.; Kheiri, S. Comparison of vaginal ointment of honey and clotrimazole for treatment of vulvovaginal candidiasis: A random clinical trial. J. Med. Mycol. 2017, 27, 494–500. [Google Scholar] [CrossRef]
- Okeniyi, J.A.; Olubanjo, O.O.; Ogunlesi, T.A.; Oyelami, O.A. Comparison of Healing of Incised Abscess Wounds with Honey and EUSOL Dressing. J. Altern. Complement. Med. 2005, 11, 511–513. [Google Scholar] [CrossRef]
- Lavaf, M.; Simbar, M.; Mojab, F.; Majd, H.A.; Samimi, M. Comparison of honey and phenytoin (PHT) cream effects on intensity of pain and episiotomy wound healing in nulliparous women. J. Complement. Integr. Med. 2018, 15, 20160139. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, M.B.; Baig, M. A randomized, controlled clinical trial of honey-impregnated dressing for treating diabetic foot ulcer. J. Coll. Physicians Surg. Pak. 2015, 25, 721–725. [Google Scholar]
- Moghazy, A.M.; Shams, M.E.; Adly, O.A.; Abbas, A.H.; El-Badawy, M.A.; Elsakka, D.M.; Hassan, S.A.; Abdelmohsen, W.S.; Ali, O.S.; Mohamed, B.A. The clinical and cost effectiveness of bee honey dressing in the treatment of diabetic foot ulcers. Diabetes Res. Clin. Pract. 2010, 89, 276–281. [Google Scholar] [CrossRef]
- Shukrimi, A.; Sulaiman, A.R.; Halim, A.Y.; Azril, A. A comparative study between honey and povidone iodine as dressing solution for Wagner type II diabetic foot ulcers. Med. J. Malays. 2008, 63, 44–46. [Google Scholar]
- Schramm, D.D.; Karim, M.; Schrader, H.R.; Holt, R.R.; Cardetti, M.; Keen, C.L. Honey with High Levels of Antioxidants Can Provide Protection to Healthy Human Subjects. J. Agric. Food Chem. 2003, 51, 1732–1735. [Google Scholar] [CrossRef]
- Craig, J.P.; Cruzat, A.; Cheung, I.M.Y.; Watters, G.A.; Wang, M.T.M. Randomized masked trial of the clinical efficacy of MGO Manuka Honey microemulsion eye cream for the treatment of blepharitis. Ocul. Surf. 2020, 18, 170–177. [Google Scholar] [CrossRef]
- Rajan, T.V.; Tennen, H.; Lindquist, R.L.; Cohen, L.; Clive, J. Effect of ingestion of honey on symptoms of rhinoconjunctivitis. Ann. Allergy Asthma Immunol. 2002, 88, 198–203. [Google Scholar] [CrossRef]
- Wallace, A.; Eady, S.; Miles, M.; Martin, H.; McLachlan, A.; Rodier, M.; Willis, J.; Scott, R.; Sutherland, J. Demonstrating the safety of manuka honey UMF® 20+ in a human clinical trial with healthy individuals. Br. J. Nutr. 2010, 103, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Amiri Farahani, L.; Hasanpoor-Azghdy, S.B.; Kasraei, H.; Heidari, T. Comparison of the effect of honey and mefenamic acid on the severity of pain in women with primary dysmenorrhea. Arch. Gynecol. Obstet. 2017, 296, 277–283. [Google Scholar] [CrossRef]
- Chepulis, L.; Starkey, N. The Long-Term Effects of Feeding Honey Compared with Sucrose and a Sugar-Free Diet on Weight Gain, Lipid Profiles, and DEXA Measurements in Rats. J. Food Sci. 2008, 73, H1–H7. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Effects of Honey on the Urinary Total Nitrite and Prostaglandins Concentration. Int. Urol. Nephrol. 2005, 37, 107–111. [Google Scholar] [CrossRef]
- Smukler, S.R.; Tang, L.; Wheeler, M.B.; Salapatek, A.M.F. Exogenous Nitric Oxide and Endogenous Glucose-Stimulated β-Cell Nitric Oxide Augment Insulin Release. Diabetes 2002, 51, 3450–3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Waili, N.S. Effects of Daily Consumption of Honey Solution on Hematological Indices and Blood Levels of Minerals and Enzymes in Normal Individuals. J. Med. Food 2004, 6, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.N.; Geloneze, B.; Tambascia, M.A.; Lerário, A.C.; Halpern, A.; Cozzolino, S.M.F. Effect of Zinc Supplementation on Serum Leptin Levels and Insulin Resistance of Obese Women. Biol. Trace Elem. Res. 2006, 112, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance, and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafian, M.; Ebrahim-Habibi, A.; Yaghmaei, P.; Parivar, K.; Larijani, B. Core structure of flavonoids precursor as an antihyperglycemic and antihyperlipidemic agent: An in vivo study in rats. Acta Biochim. Pol. 2010, 57, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, M.; Orhan, D.D.; Orhan, N.; Sezik, E.; Yeşilada, E. A Study of Antidiabetic and Antioxidant Effects of Helichrysum graveolens Capitulums in Streptozotocin-Induced Diabetic Rats. J. Med. Food 2007, 10, 396–400. [Google Scholar] [CrossRef]
- Li, W.; Dai, R.-J.; Yu, Y.-H.; Li, L.; Wu, C.-M.; Luan, W.-W.; Meng, W.-W.; Zhang, X.-S.; Deng, Y.-L. Antihyperglycemic Effect of Cephalotaxus sinensis Leaves and GLUT-4 Translocation Facilitating Activity of Its Flavonoid Constituents. Biol. Pharm. Bull. 2007, 30, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Balomajumder, C.; Roy, P. Hypoglycemic and hypolipidemic effects of flavonoid rich extract from Eugenia jambolana seeds on streptozotocin induced diabetic rats. Food Chem. Toxicol. 2008, 46, 2376–2383. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kwon, C.-S.; Son, K.H. Inhibition of Alpha-glucosidase and Amylase by Luteolin, a Flavonoid. Biosci. Biotechnol. Biochem. 2000, 64, 2458–2461. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-Glucosidase and α-Amylase by Flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for Controlling Starch Digestion: Structural Requirements for Inhibiting Human α-Amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [Green Version]
- Gheldof, N.; Engeseth, N.J. Antioxidant Capacity of Honeys from Various Floral Sources Based on the Determination of Oxygen Radical Absorbance Capacity and Inhibition of in Vitro Lipoprotein Oxidation in Human Serum Samples. J. Agric. Food Chem. 2002, 50, 3050–3055. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Vásquez, A. Detection and Identification of a Novel Lactic Acid Bacterial Flora Within the Honey Stomach of the Honeybee Apis mellifera. Curr. Microbiol. 2008, 57, 356–363. [Google Scholar] [CrossRef]
- Panero, F.; Novelli, G.; Zucco, C.; Fornengo, P.; Perotto, M.; Segre, O.; Grassi, G.; Cavallo-Perin, P.; Bruno, G. Fasting Plasma C-Peptide and Micro- and Macrovascular Complications in a Large Clinic-Based Cohort of Type 1 Diabetic Patients. Diabetes Care 2009, 32, 301–305. [Google Scholar] [CrossRef] [Green Version]
- Buschard, K. What causes type 1 diabetes? Lessons from animal models. Apmis 2011, 119, 1–19. [Google Scholar] [CrossRef]
- Shixian, Q.; VanCrey, B.; Shi, J.; Kakuda, Y.; Jiang, Y.; Cardoso, G.A.; Salgado, J.M.; Cesar, M.d.C.; Donado-Pestana, C.M.; Bajerska, J.; et al. Green Tea Extract Thermogenesis-Induced Weight Loss by Epigallocatechin Gallate Inhibition of Catechol-O-Methyltransferase. J. Med. Food 2007, 9, 451–458. [Google Scholar] [CrossRef]
- Samdariya, S.; Lewis, S.; Kauser, H.; Ahmed, I.; Kumar, D. A randomized controlled trial evaluating the role of honey in reducing pain due to radiation induced mucositis in head and neck cancer patients. Indian J. Palliat. Care 2015, 21, 268–273. [Google Scholar] [CrossRef]
- Al Jaouni, S.K.; Al Muhayawi, M.S.; Hussein, A.; Elfiki, I.; Al-Raddadi, R.; Al Muhayawi, S.M.; Almasaudi, S.; Kamal, M.A.; Harakeh, S. Effects of Honey on Oral Mucositis among Pediatric Cancer Patients Undergoing Chemo/Radiotherapy Treatment at King Abdulaziz University Hospital in Jeddah, Kingdom of Saudi Arabia. Evid.-Based Complement. Altern. Med. 2017, 2017, 5861024. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Sánchez-Lara, K.; Ugalde-Morales, E.; Motola-Kuba, D.; Green, D. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br. J. Nutr. 2013, 109, 894–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Co, J.L.; Mejia, M.B.A.; Que, J.C.; Dizon, J.M.R. Effectiveness of honey on radiation-induced oral mucositis, time to mucositis, weight loss, and treatment interruptions among patients with head and neck malignancies: A meta-analysis and systematic review of literature. Head Neck 2016, 38, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Bendall, L.J.; Bradstock, K.F. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014, 25, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eccles, R. Mechanisms of the placebo effect of sweet cough syrups. Respir. Physiol. Neurobiol. 2006, 152, 340–348. [Google Scholar] [CrossRef]
- Brook, I. Infant botulism. J. Perinatol. 2007, 27, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Godart, V.; Dan, B.; Mascart, G.; Fikri, Y.; Dierick, K.; Lepage, P. Botulisme infantile après exposition à du miel. Arch. Pediatr. 2014, 21, 628–631. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to honey and “respiratory health through presence of antioxidant phytochemicals” (ID 1161), “the unique composition and ratio of effective substances adds energy to the human body” (ID 3188), and “it stimulates the whole metabolism and the immune system” (ID 3189) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1484. [Google Scholar] [CrossRef]

| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | Reference |
|---|---|---|---|---|---|---|
| Natural honey | 70 g/d vs. sucrose | 6 w | Healthy males 25.51 ± 1.63 years | TG | ↓ *,a | [12] |
| TC | ↓ *,a | |||||
| LDL | ↓ *,a | |||||
| HDL | ↑ *,a | |||||
| Natural, unprocessed honey purchased from Ilyas Traders, Charsadda, Khyber Pakhtunkhwa, Pakistan | Diet + 70 g/d vs. diet | 1 m | Healthy Pakistani males 20.13 ± 0.14 years | Increase in FBG | ↓ a | [13] |
| TG | ↓ a | |||||
| TC | ↓ *,a | |||||
| LDL | ↓ *,a | |||||
| HDL | ↑ *,a | |||||
| Mixture of four types of clover honey obtained from Golden Heritage Foods, Smitty Bee Honey, Millers Honey Company, and Marshall’s Farm Natural Honey | 1.5 g/kg/d honey vs. sucrose | 1 m | Healthy subjects 32.9 ± 1.7 years | FBI | = | [14] |
| TC | ↓ | |||||
| LDL | ↓ | |||||
| HDL | ↓ | |||||
| Iranian natural honey | 70 g/d vs. sucrose | 1 m | Overweight or obese subjects 42.6 ± 8.6 years | TG | ↓ b | [15] |
| TC | ↓ | |||||
| LDL | ↓ | |||||
| HDL | ↑ | |||||
| FBG | ↓ * | |||||
| CRP | ↓ b | |||||
| BW | ↓ | |||||
| BF | ↓ | |||||
| BMI | ↓ * | |||||
| Wild flowers-forest-thyme honey produced by Attiki | Diet + 15 g/d vs. diet + marmelade | 6 m | Obese girls 10.55 ± 0.34 years | BMI | ↓ | [16] |
| TG | ↓ | |||||
| TC | ↑ | |||||
| LDL | ↑ | |||||
| HDL | ↑ | |||||
| OGT | ↑ | |||||
| Dutch Gold Honey (honey from different floral sources and geographic origin) | 50 g/d vs. sucrose or corn syrup | 2 w | Glucose-tolerant with overweight or obesity 38.9 ± 3.6 years | BW | ↑ | |
| BMI | = | [17] | ||||
| SBP | = | |||||
| DBP | = | |||||
| TG | ↑ * | |||||
| TC | ↑ | |||||
| LDL | = | |||||
| HDL | = | |||||
| FBG | ↓ | |||||
| FBI | ↓ | |||||
| Glucose-intolerant with overweight or obesity 52.1 ± 2.7 years | BW | ↑ | ||||
| BMI | ↑ | |||||
| SBP | ↑ | |||||
| DBP | = | |||||
| TG | ↑ | |||||
| TC | ↑ | |||||
| LDL | ↑ | |||||
| HDL | ↑ | |||||
| FBG | ↑ | |||||
| FBI | ↑ | |||||
| Tualang sterilized honey supplied by Federal Agricultural Marketing Authorities (FAMA), Malaysia | 20 g/d vs. honey cocktail | 12 m | Post-menopausal healthy and diabetic women 58.1 ± 3.7 years | SBP | ↓ | [18] |
| DBP | ↓ a | |||||
| TC | ↓ | |||||
| LDL | ↑ | |||||
| HDL | ↓ | |||||
| TG | ↑ | |||||
| FBG | ↓ a | |||||
| BMI | ↑ | |||||
| BF | ↑ | |||||
| WC | ↑ | |||||
| Iranian natural unprocessed honey collected from Samans kandeh, Neka, Sari City | 1 g/kg/d first 2 w 1.5 g/kg/d second 2 w 2 g/kg/d third 2 w 2.5 g/kg/d last 2 w | 8 w | Type II diabetes 57.2 ± 8.4 years | BW | ↓ *,a | [19] |
| FBG | ↓ | |||||
| HbA1c | ↑ * | |||||
| TG | ↓ * | |||||
| TC | ↓ * | |||||
| LDL | ↓ * | |||||
| HDL | ↑ * | |||||
| Unprocessed Egyptian clover honey supplied by a beekeeper | 0.5 mL/kg/d | Crossover study Two 12 w intervention periods | Type I diabetes 4.7 ± 4.28 years | SSFT | ↓ * | [20] |
| MC | ↓ | |||||
| TSFT | ↓ | |||||
| FBG | ↓ * | |||||
| TG | ↓ * | |||||
| TC | ↓ * | |||||
| LDL | ↓ * | |||||
| HDL | ↑ * | |||||
| C-peptide | ↑ * | |||||
| HbA1C | ↓ * | |||||
| Natural honey | 75 g/d | 15 d | Healthy subjects | FBG | ↓ | [21] |
| TG | ↓ | |||||
| TC | ↓ | |||||
| LDL | ↓ | |||||
| HDL | ↑ | |||||
| CRP | ↓ | |||||
| Patients with hyperlipidemia | TC | ↓ * | ||||
| LDL | ↓ | |||||
| CRP | ↓ * | |||||
| Mixed blossom honey from Europe, Central America, and South America | 75 g/d vs. sugar solution | 2 w | Subjects with hypercholesterolemia 35–86 years | TG | ↑ | [22] |
| TC | ↓ | |||||
| LDL | ↑ | |||||
| HDL | ↓ |
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | Reference |
|---|---|---|---|---|---|---|
| Mixture of four types of clover honey obtained from Golden Heritage Foods, Smitty Bee Honey, Millers Honey Company, and Marshall’s Farm Natural Honey | 1.5 g/kg/d honey vs. sucrose | 1 m | Healthy subjects 24–57 years | Increase in energy intake | ↓ a | [14] |
| Increase in carbohydrate intake | ↓ a | |||||
| Increase in sugar intake | ↓ a | |||||
| Pure clover honey | 42.7 g vs. 35.5 g of sucrose | 1 d | Healthy women 21.8 ± 2.9 years | Increase in post-prandial glucose | ↓ a | |
| Increase in post-prandial insulin | ↑ | |||||
| Post-prandial leptin | ↑ | [26] | ||||
| Post-prandial ghrelin | ↓ | |||||
| Post-prandial peptide YY | ↑ | |||||
| Hunger rate | ↓ | |||||
| Satiety rate | ↑ a | |||||
| Thermogenesis | ↑ | |||||
| Energy intake | ↑ | |||||
| Carbohydrate intake | ↑ | |||||
| Sugar intake | ↓ | |||||
| Iranian natural unprocessed honey collected from Samans kandeh, Neka, Sari City | 1 g/kg/d first 2 w 1.5 g/kg/d 2 w 2 g/kg/d 2 w 2.5 g/kg/d last 2 w | 8 w | Type II diabetes 57.2 ± 8.4 years | Energy intake | ↓ | [19] |
| Energy from protein | ↓ | |||||
| Energy from carbohydrate | ↑ | |||||
| Energy from fat | ↓ | |||||
| Sugar intake | ↑ |
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | References |
|---|---|---|---|---|---|---|
| Freshly harvested Nigerian citrus (Citrus sinensis Osbeck) honey from the delta region of the River Niger | 0.5 mL/kg of ethanol + 1 mL/kg of honey | 1 d | Healthy subjects 25–35 years | Blood alcohol clearance rate | ↓ * | [27] |
| Intoxication time | ↓ * | |||||
| Intoxication degree | ↓ | |||||
| Freshly harvested Nigerian citrus (Citrus sinensis Osbeck) honey from the delta region of the River Niger | 0.5 g/kg of ethanol + 1.25 mL/kg of honey | 1 d | Healthy men 23.6 ± 7.4 years | Intoxication time | ↓ * | [28] |
| Intoxication degree | ↓ * | |||||
| TG | ↑ * | |||||
| Blood pressure | ↑ |
| Honey | Dose | Duration | Subjects | Physiological Parameter | Effect | Reference |
|---|---|---|---|---|---|---|
| Low- and high- antioxidant buckwheat honey from the Dutch Gold company | 1.5 g/kg | 6 h | Healthy subjects 25.55 ± 2.30 years | Plasma phenolic concentration | ↑ * | [56] |
| Plasma antioxidant capacity | ↑ * | |||||
| Plasma reducing capacity | ↑ * | |||||
| Manuka honey from New Zealand | Manuka honey microemulsion cream 0.5–1 cm Once a day | 3 m | Patients with blepharitis 60 ± 12 years | Dry eye symptomology | ↓ a | [57] |
| Tear film quality | ↑ a | |||||
| Ocular surface quality | ↑ a | |||||
| Microbial burden | ↓ a | |||||
| Local unpasteurized honey from Honeycomb Apiairies, Bristol and filtered pasteurized clover honey from Dutch Gold Honey Inc, Lancaster | 1 tablespoonful/d | 30 w | Patients with allergic rhinoconjunctivitis 45.3 years | Symptoms of rhinoconjunctivitis | = | [58] |
| Multiflora honey and Manuka honey UMF 20+, both produced by Comvita New Zealand Ltd. | 20 g/d | Crossover 4 w each period | Healthy subjects 42–64 years | IgE level | ↑ | [59] |
| Gut bacterial | = | |||||
| Astragalus honey made in Ashtian Region of Iran | 1.2 g/kg from the 15th day to the onset of menstruation | Crossover 2 m each period | Female students with dysmenorrhea 22.01 ± 1.78 years | Pain | = | [60] |
| Amount of bleeding | = | |||||
| Satisfaction | = |
| Condition | Subjects | Parameter | Effect | References |
|---|---|---|---|---|
| Cardiovascular risk factors | Healthy subjects Diabetic subjects Subjects with hyperlipidaemia | FBG | ↓ | [12,13,18,19,20,21] |
| TG | ↓ | |||
| TC | ↓ | |||
| LDL | ↓ | |||
| HDL | ↑ | |||
| Glucose tolerance | Healthy subjects Diabetic subjects | Increase in BGL | ↓ | [21,23,24,25] |
| Increase in BIL | ↓ | |||
| Alcohol metabolism | Healthy subjects | Intoxication time | ↓ | [27,28] |
| Cancer | Patients with acute myeloid leukaemia Patients with head and neck cancer | Mucositis severity | ↓ | [29,30,31,32,33,34,39] |
| Body weight | ↑ | |||
| URTIs | Children with URTIs | Cough frequency and severity | ↓ | [42,43,44,45,46] |
| Combined symptom score | ↓ | |||
| Sleep quality | ↑ | |||
| Parent’s sleep quality | ↑ | |||
| Wounds | Children with pyomyositis abscesses Women with episiotomy wound Patients with diabetes | Wound healing | ↑ | [51,52,53,54] |
| Discharge | ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. https://doi.org/10.3390/nu15133056
Palma-Morales M, Huertas JR, Rodríguez-Pérez C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients. 2023; 15(13):3056. https://doi.org/10.3390/nu15133056
Chicago/Turabian StylePalma-Morales, Marta, Jesús R. Huertas, and Celia Rodríguez-Pérez. 2023. "A Comprehensive Review of the Effect of Honey on Human Health" Nutrients 15, no. 13: 3056. https://doi.org/10.3390/nu15133056
APA StylePalma-Morales, M., Huertas, J. R., & Rodríguez-Pérez, C. (2023). A Comprehensive Review of the Effect of Honey on Human Health. Nutrients, 15(13), 3056. https://doi.org/10.3390/nu15133056