Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Euscaphis japonica (Thunb.) Kanitz Fruit Extract
2.2. Cell Culture
2.3. Adipogenic Differentiation of 3T3-L1 Preadipocytes
2.4. Cell Viability Assay
2.5. Oil Red O Staining
2.6. Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.7. Western Blot Assay
2.8. Cell Cycle Assay
2.9. Statistical Analysis
3. Results
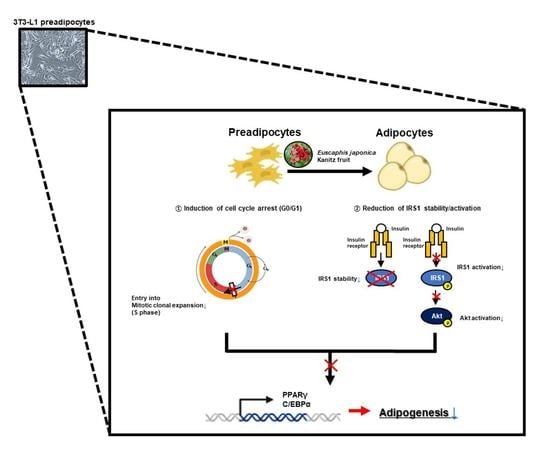
3.1. EJF Extract Inhibits Adipogenic Differentiation in 3T3-L1 Preadipocytes
3.2. The Inhibitory Effect of EJF on Adipogenesis Is Most Critical in the Early Stage of Differentiation in 3T3-L1 Preadipocytes
- ⬩
- Early stage: After post-confluence, treatment of MDI initiates differentiation. This stage is characterized by the presence of growth-arrested preadipocytes and mitotic clonal expansion (MCE) phase.
- ⬩
- Middle stage: After 2 days of MDI treatment, transitioning to media containing only insulin leads to entering the middle stage. In this stage, adipogenic gene expression is initiated, and the accumulation of lipid droplets begins.
- ⬩
- Late stage: After the 4th of differentiation, adipocytes enter the maturation stage. This stage is characterized by the formation of mature lipid-filled adipocytes.
3.3. EJF Inhibits the Early Stage of Adipogenic Differentiation by Inhibiting MDI-Induced Cell Cycle Progression in 3T3-L1 Preadipocytes
- ⬩
- Cell cycle distribution: The NC group induced cell cycle arrest in G0/G1 phase, and the MDI (+) group initiated both cell cycle progression and MCE (S phase). However, after EJF treatment, the percentage of cells in S phase decreased and the percentage of cells in G0/G1 increased (Figure 3A,B).
- ⬩
- CDK4, Cyclin D: A significant decrease in the expression levels of CDK4 and cyclin D was observed at 16 h in the group treated with EJF compared to that in the group without EJF treatment (Figure 3C–F).
- ⬩
- p27 KIP1: The MDI (+) group exhibited a decrease in the expression of p27KIP1 compared to the NC group due to the resumption of the cell cycle. However, treatment with EJF significantly increased the expression of p27KIP1, and this observation remained consistent regardless of the time point (Figure 3C,D,G).
3.4. EJF Attenuates IRS1 Stability and Inhibits Its Activation in 3T3-L1 Preadipocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stavridou, A.; Kapsali, E.; Panagouli, E.; Thirios, A.; Polychronis, K.; Bacopoulou, F.; Psaltopoulou, T.; Tsolia, M.; Sergentanis, T.N.; Tsitsika, A. Obesity in children and adolescents during COVID-19 pandemic. Children 2021, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Suwa, A.; Kurama, T.; Shimokawa, T. Adipocyte hyperplasia and RMI1 in the treatment of obesity. FEBS J. 2011, 278, 565–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freemark, M. Childhood obesity in the modern age: Global trends, determinants, complications, and costs. In Pediatric Obesity; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–24. [Google Scholar]
- Daniels, S. Complications of obesity in children and adolescents. Int. J. Obes. 2009, 33, S60–S65. [Google Scholar] [CrossRef] [Green Version]
- Robinson, G.A.; Geier, M.; Rizzolo, D.; Sedrak, M. Childhood obesity: Complications, prevention strategies, treatment. J. Am. Acad. Physician Assist. 2011, 24, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.J. Pharmacological Treatment of Obesity. In Endocrinology and Diabetes; Springer: Berlin/Heidelberg, Germany, 2008; p. 23. [Google Scholar]
- Ioannides-Demos, L.L.; Proietto, J.; Tonkin, A.M.; McNeil, J.J. Safety of drug therapies used for weight loss and treatment of obesity. Drug Saf. 2006, 29, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, N.; Yadav, N.; Sharma, S.K. Natural products: A safest approach for obesity. Chin. J. Integr. Med. 2012, 18, 473–480. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural products with anti-obesity effects and different mechanisms of action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Vermaak, I.; Viljoen, A.M.; Hamman, J.H. Natural products in anti-obesity therapy. Nat. Prod. Rep. 2011, 28, 1493–1533. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.H.; Lee, T.S.; Oh, W.K.; Kim, D.S.; Kim, J.B. Selective LXRα inhibitory effects observed in plant extracts of MEH184 (Parthenocissua tricuspidata) and MEH185 (Euscaphis japonica). Biochem. Biophys. Res. Commun. 2006, 349, 513–518. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Fernández-Real, J.M. Adipocyte differentiation. In Adipose Tissue Biology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 69–90. [Google Scholar]
- Knutson, V.P. 3T3-L1 adipocytes as a cell culture model of insulin resistance. In Vitro Cell. Dev. Biol. Anim. 1997, 33, 77–81. [Google Scholar] [CrossRef]
- Chang, E.; Kim, C.Y. Natural products and obesity: A focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules 2019, 24, 1157. [Google Scholar] [CrossRef] [Green Version]
- Fajas, L. Adipogenesis: A cross-talk between cell proliferation and cell differentiation. Ann. Med. 2003, 35, 79–85. [Google Scholar] [CrossRef]
- Park, H.; Cho, J.A.; Lim, E.H.; Lee, C.W.; Lee, S.H.; Seo, S.W.; Yang, D.Y.; Lee, K.W. Cell cycle regulators are critical for maintaining the differentiation potential and immaturity in adipogenesis of adipose-derived stem cells. Differentiation 2011, 82, 136–143. [Google Scholar] [CrossRef]
- Meng, Q.; Qi, X.; Chao, Y.; Chen, Q.; Cheng, P.; Yu, X.; Kuai, M.; Wu, J.; Li, W.; Zhang, Q. IRS1/PI3K/AKT pathway signal involved in the regulation of glycolipid metabolic abnormalities by Mulberry (Morus alba L.) leaf extracts in 3T3-L1 adipocytes. Chin. Med. 2020, 15, 1. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Yu, B.; Kakino, M.; Fujimoto, H.; Ando, Y.; Hakuno, F.; Takahashi, S.-I. A novel IRS-1-associated protein, DGKζ regulates GLUT4 translocation in 3T3-L1 adipocytes. Sci. Rep. 2016, 6, 35438. [Google Scholar] [CrossRef] [Green Version]
- Maki, C.; Funakoshi-Tago, M.; Aoyagi, R.; Ueda, F.; Kimura, M.; Kobata, K.; Tago, K.; Tamura, H. Coffee extract inhibits adipogenesis in 3T3-L1 preadipocyes by interrupting insulin signaling through the downregulation of IRS1. PLoS ONE 2017, 12, e0173264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, Y.; Inamitsu, T.; Chida, K.; Iemura, S.-I.; Natsume, T.; Maeda, T.; Hakuno, F.; Takahashi, S.-I. Serine phosphorylation by mTORC1 promotes IRS-1 degradation through SCFβ-TRCP E3 ubiquitin ligase. iScience 2018, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Spiegelman, B.; Puigserver, P.; Wu, Z. Regulation of adipogenesis and energy balance by PPARγ and PGC-1. Int. J. Obes. 2000, 24, S8–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell. Biol. 2019, 39, e00601–e00618. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, K.; Chatterjee, T.; Chowdhury, S.; Sengupta, S.; Bhattacharyya, M. Adiponectin genetic variant and expression coupled with lipid peroxidation reveal new signatures in diabetic dyslipidemia. Biochem. Genet. 2021, 59, 781–798. [Google Scholar] [CrossRef]
- Maeda, H.; Matsuo, Y.; Tanaka, T.; Kouno, I. Euscaphinin, a New Ellagitannin Dimer from Euscaphis japonica (T HUNB.) K ANITZ. Chem. Pharm. Bull. 2009, 57, 421–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.-J.; Cheng, J.-J.; Liao, C.-C.; Cheng, H.-L.; Huang, H.-T.; Kuo, L.-M.Y.; Kuo, Y.-H. Triterpene acids from Euscaphis japonica and assessment of their cytotoxic and anti-NO activities. Planta Med. 2012, 78, 1584–1590. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, S.; Lu, Y.; Li, L.; Shi, Y.; Lei, Y.; Yang, X.; Wu, Z. Effect of a hexacyclic triterpenic acid from Euscaphis japonica on the oleic acid induced HepG2 cellular model of non-alcoholic fatty liver disease. Med. Chem. Res. 2022, 31, 2209–2219. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, S.S.; Park, M.H.; Jang, H.; Lee, Y.H.; Khim, K.W.; Oh, S.R.; Park, J.; Ryu, H.W.; Choi, J.H. Broussonetia papyrifera root bark extract exhibits anti-inflammatory effects on adipose tissue and improves insulin sensitivity potentially via AMPK activation. Nutrients 2020, 12, 773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guru, A.; Issac, P.K.; Velayutham, M.; Saraswathi, N.; Arshad, A.; Arockiaraj, J. Molecular mechanism of down-regulating adipogenic transcription factors in 3T3-L1 adipocyte cells by bioactive anti-adipogenic compounds. Mol. Biol. Rep. 2021, 48, 743–761. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Accession No. | Sequence | |
|---|---|---|---|
| Adipoq | NM_009605 | Forward | 5′-ACCTACGACCAGTATCAGGAAAAG-3′ |
| Reverse | 3′-ACTAAGCTGAAAGTGTGTCGACTG-5′ | ||
| C/ebpα | NM_001287523 | Forward | 5′-TTACAACAGGCCAGGTTTCC-3′ |
| Reverse | 3′-GGCTGGCGACATACAGATCA-5′ | ||
| Pparγ | AB644275 | Forward | 5′-TTTTCAAGGGTGCCAGTTTC-3′ |
| Reverse | 3′-AATCCTTGGCCCTCTGAGAT-5′ | ||
| β-actin | EF095208 | Forward | 5′-GACAACGGCTCCGGCATGTGCAAAG-3′ |
| Reverse | 3′-TTCACGGTTGGCCTTAGGGTTCAG-5′ |
| Gene Name | Company | Product No. | IgG | Conc. |
|---|---|---|---|---|
| Primary antibody | ||||
| PPARγ | SCBT | sc-7273 | M | 1:500 |
| C/EBPα | CST | 2295S | R | 1:1000 |
| IRS1 | CST | 2390S | R | 1:1000 |
| p-IRS1 | Invitrogen | 2103503 | R | 1:1000 |
| Akt | CST | 4691S | R | 1:1000 |
| p-Akt | CST | 4060S | R | 1:1000 |
| β-actin | SCBT | sc-47778 | M | 1:500 |
| β-tubulin | Abcam | ab179513 | R | 1:1000 |
| Secondary antibody | ||||
| Mouse | CST | 7076S | 1:2000 | |
| Rabbit | CST | 7074S | 1:3000 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.; Park, J.; Nam, J.-O. Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation. Nutrients 2023, 15, 3078. https://doi.org/10.3390/nu15143078
Lee E, Park J, Nam J-O. Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation. Nutrients. 2023; 15(14):3078. https://doi.org/10.3390/nu15143078
Chicago/Turabian StyleLee, Eunbi, Juhye Park, and Ju-Ock Nam. 2023. "Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation" Nutrients 15, no. 14: 3078. https://doi.org/10.3390/nu15143078
APA StyleLee, E., Park, J., & Nam, J. -O. (2023). Euscaphis japonica Kanitz Fruit Exerts Antiobesity Effects by Inhibiting the Early Stage of Adipogenic Differentiation. Nutrients, 15(14), 3078. https://doi.org/10.3390/nu15143078