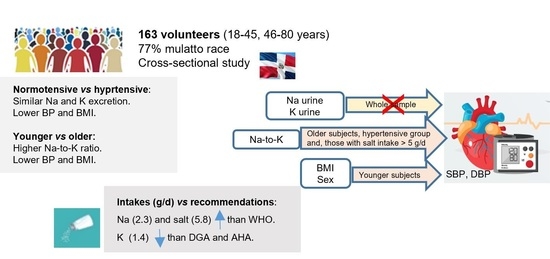
Assessment of 24 h Sodium and Potassium Urinary Excretion in Normotensive and Hypertensive Dominican Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.1.1. Anthropometric and Blood Pressure Measurements
2.1.2. Urine and Blood Samples
2.1.3. Statistical Analysis
3. Results
4. Discussion
4.1. Urinary Sodium and Potassium Excretion
4.1.1. Estimated Sodium, Salt, and Potassium Intake: Comparison with Recommended Intake
4.1.2. Strengths and Limitations of This Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McGuire, S. Institute of Medicine. 2013. Sodium intake in populations: Assessment of evidence. Washington, DC: The National Academies Press. 2013. Adv. Nutr. 2014, 5, 19–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.W.; Baqar, S.; Jerums, F.; Ekinci, E.I. Sodium and its role in cardiovascular disease-the debate continues. Front. Endocrinol. 2016, 7, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; He, F.J.; Sun, Q.; Yuan, C.; Kieneker, L.M.; Curhan, G.C.; MacGregor, G.A.; Bakker, S.J.; Campbell, N.R.; Wang, M.; et al. Urinary sodium and potassium excretion and cardiovascular risk. N. Engl. J. Med. 2022, 386, 252–263. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. Br. Med. J. 2013, 3, 1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrissey, E.; Giltinan, M.; Kehoe, L.; Nugent, A.P.; McNulty, B.A.; Flynn, A.; Walton, J. Sodium and potassium intakes and their ratio in adults (18-90 y): Findings from the Irish National Adult Nutrition Survey. Nutrients 2020, 12, 938. [Google Scholar] [CrossRef] [Green Version]
- Langford, H.G. Dietary potassium and hypertension: Epidemiologic data. Ann. Intern. Med. 1983, 98, 770–772. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium collaborative Research Group. N. Engl. J. Med. 2001, 34, 3–10. [Google Scholar] [CrossRef]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [CrossRef] [Green Version]
- Perez, V.; Chang, E.T. Sodium-to-potassium ratio and blood pressure, hypertension, and related factors. Adv. Nutr. 2014, 5, 712–741. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kwock, C.K.; Yan, Y.J. The effect of the sodium to potassium ratio on hypertension prevalence: A propensity score matching approach. Nutrients 2016, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Iwahori, T.; Miura, K.; Ueshima, H. Time to consider use of the sodium-to-potassium ratio for practical sodium reduction and potassium increase. Nutrients 2017, 8, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Athanasatou, A.; Kandyliari, A.; Malisova, O.; Pepa, A.; Kapsokefalou, M. Sodium and Potassium Intake from Food Diaries and 24-h Urine Collections from 7 Days in a Sample of Healthy Greek Adults. Front. Nutr. 2018, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohara-Shitama, Y.; Adachi, H.; Enomoto, M.; Fukami, A.; Kumagai, E.; Nakamura, S.; Kono, S.; Morikawa, N.; Nakao, E.; Sakaue, A.; et al. Twenty-four-hour urinary potassium excretion, but not sodium excretion, is associated with all-cause mortality in a general population. J. Am. Heart Assoc. 2018, 7, e007369. [Google Scholar] [CrossRef] [Green Version]
- Bhagavathula, A.S.; Refaat, S.A.; Bentley, B.L.; Rahmani, J. Association between intake of sodium, potassium, sodium-to-potassium ratio, and blood pressure among US adults. Int. J. Vitam. Nutr. Res. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.; Gardener, H.; Cespedes, S.; Cheung, Y.K.; Sacco, R.L.; Elkind, M.S.V. Dietary sodium to potassium ratio and risk of stroke in a multiethnic urban population. The Northern Manhattan Study. Stroke 2017, 48, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Huang, X.; Liu, X.; Wang, L.; Chen, Z.; Ortega, D.; Chen, L.; Sun, J.; Hatsukami, T.S.; Yuan, C.; et al. Urinary sodium and potassium excretion and cerebrovascular health: A multimodal imaging study. Eur. J. Nutr. 2021, 60, 4555–4563. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Hypertension. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/hypertension (accessed on 20 February 2023).
- American Diabetes Association; Bantle, J.P.; Wylie-Rosett, J.; Albright, A.L.; Apovian, C.M.; Clark, N.G.; Franz, M.J.; Hoogwerf, B.J.; Lichtenstein, A.H.; Mayer-Davis, E.; et al. Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care 2008, 31, S61–S78. [Google Scholar]
- Lloyd-Jones, D.M.; Hong, Y.; Labarthe, D.; Mozaffarian, D.; Appel, L.J.; Van Horn, L.; Greenlund, K.; Daniels, S.; Nichol, G.; Tomaselli, G.F.; et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010, 121, 586–613. [Google Scholar] [CrossRef] [Green Version]
- Kidney Disease: Improving Global Outcomes (KDIGO). CKD Work Group, KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150.
- WHO. Sodium Intake for Adults and Children; WHO: Geneve, Switzerland, 2012; Available online: https://apps.who.int/iris/bitstream/handle/10665/77985/9789241504836_eng.pdf (accessed on 20 February 2023).
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans, 8th ed.; U.S. Department of Health and Human Services and U.S. Department of Agriculture: Washington, DC, USA, 2015. Available online: https://health.gov/our-work/food-nutrition/previous-dietary-guidelines/2015 (accessed on 20 February 2023).
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J. Scientific Opinion on the dietary reference values for sodium. EFSA J. 2019, 17, 5778. [Google Scholar]
- AHA—American Heart Association. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/sodium/how-much-sodium-should-i-eat-per-day (accessed on 20 February 2023).
- Aparicio, A.; Rodríguez-Rodríguez, E.; Cuadrado-Soto, E.; Navia, B.; López-Sobaler, A.M.; Ortega, R.M. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7–11 years. Eur. J. Nutr. 2017, 56, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Ortega, R.M.; López-Sobaler, A.M.; Ballesteros, J.M.; Pérez-Farinós, N.; Rodriguez-Rodriguez, E.; Aparicio, A.; Perea, J.M.; Andrés, P. Estimation of salt intake by 24 h urinary sodium excretion in a representative sample of Spanish adults. Br. J. Nutr. 2011, 105, 787–794. [Google Scholar] [CrossRef]
- World Health Organization. Reducing Salt Intake in Populations: Report of a WHO Forum and Technical Meeting, 5–7 October 2006, Paris, France; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- WHO/PAHO Regional Expert Group for Cardiovascular Disease Prevention through Population-Wide Dietary Salt Reduction. Protocol for Population Level Sodium Determination in 24-Hour Urine Samples. May 2010. Available online: https://www.paho.org/hq/dmdocuments/2013/24h-urine-Protocol-eng.pdf (accessed on 20 February 2023).
- PAHO. Mapping Dietary Salt/Sodium Reduction Policies and Initiatives in the Region of the Americas; Pan American Health Organization: Washington, DC, USA, 2021. [Google Scholar]
- MISPAS—Ministerio de Salud Pública y Asistencia Social; Gobierno de la República Dominicana. 1era Jornada Nacional de Hipertensión Arterial: Prevención de Diabetes y Obesidad; Informe de Resultados; MISPAS, Ministerio de Salud Pública y Asistencia Social, Gobierno de la República Dominicana: Santo Domingo, Dominican Republic, 2021. [Google Scholar]
- PAHO (PanAmerican Health Organization). Plan of Action for the Prevention and Control of Noncommunicable Diseases in the Americas 2013–2019; OPS: Washington, DC, USA, 2014; Available online: https://www.paho.org/hq/dmdocuments/2014/NCD-SP-low.pdf (accessed on 20 February 2023).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti, E.; Azizi, M.; Burnier, M. Guía ESC/ESH 2018 sobre el diagnóstico y tratamiento de la hipertensión arterial. Rev. Esp. Cardiol. 2019, 72, 160.e1–160.e78. [Google Scholar]
- PAHO. Hypertension. Available online: https://www.paho.org/en/topics/hypertension (accessed on 20 February 2023).
- Whelton, P.K.; Caarey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/ American Heart Association and European Society of Cardiology / European Society of Hypertension Blood Pressure /Hypertension Guidelines. Eur. Heart J. 2022, 43, 3302–3311. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Consumers International. Cuestionario Sobre Conocimiento, Actitud, Comportamiento Acerca de la sal Dietética y la Salud. Available online: https://www.paho.org/hq/dmdocuments/2013/Knowledge-behaviour-questionaire-salt-Spa.pdf (accessed on 2 March 2023).
- Mosteller, R.D. Simplified calculation of body-surface area. N. Engl. J. Med. 1987, 317, 1098. [Google Scholar]
- Kawasaki, T.; Itoh, K.; Uezono, K.; Sasaki, H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin. Exp. Pharmacol. Physiol. 1993, 20, 7–14. [Google Scholar] [CrossRef]
- ISO 9001:2015; Sistema de gestión de calidad. Quality Management System. DQS Holding GmbH: Berlin, Germany, 2015; IQNet. Registration number: DE-432319 QM15.
- Oh, J.; Lee, J.; Koo, H.S.; Kim, S.; Chin, H.J. Estimated 24-hour urine sodium excretion is correlated with blood pressure in korean population: 2009-2011 Korean National Health and Nutritional Examination Survey. J. Korean Med. Sci. 2014, 29, S109–S116. [Google Scholar] [CrossRef]
- Cogswell, M.E.; Loria, C.M.; Terry, A.L.; Zhao, L.; Wang, C.-Y.; Chen, T.-C.; Wright, J.D.; Pfeiffer, C.M.; Merritt, R.; Moy, C.S.; et al. Estimated 24-hour urinary sodium and potassium excretion in US adults. JAMA 2018, 319, 1209–1220. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, P.; Li, Y.; He, F.J.; Wu, J.; Xu, J.; Zhang, X.; Li, X.; Song, J. Association of sodium, potassium and sodium-to-potassium ratio with urine albumin excretion among the general Chinese population. Nutrients 2021, 13, 3456. [Google Scholar] [CrossRef]
- Pichardo, R.; González, M.; Ramírez, W.; Escaño, F. Estudio de los factores de riesgo cardiovascular y síndrome metabólico en la República Dominicana. EFRICARD II. Rev. Domin. Cardiol. 2012, 1, 36–55. Available online: https://static.elsevier.es/cardio/static/premio_cardio/revista-dominicana-cardiologia.pdf (accessed on 20 February 2023).
- Paulino-Ramirez, R.; Mencía-Ripley, A.; Tapia-Barredo, L.; Guerrero-Martínez, S.A.; Benítez-Abreu, A. Diversidad genética en ADN mitocondrial en la República Dominicana: Implicaciones para la historia y demografía de La Española. CLÍO 2019, 88, 193–206. [Google Scholar]
- Consejo de Ministros de Salud de Centroamérica y República Dominicana (COMISCA); INCAP/OPS-OMS; Instituto de Nutrición de Centro América y Panamá/Organización Panamericana de la Salud/Organización Mundial de la Salud/Consejo de Ministros de Salud de Centroamérica y República Dominicana. Estrategia Regional Para la Reducción del Consumo de sal y Sodio en Centroamérica y República Dominicana. Enero. 2019. Available online: http://www.incap.int/index.php/es/noticias/145-estrategia-regional-para-la-reduccion-del-consumo-de-sal-y-sodio-en-centroamerica-y-republica-dominicana (accessed on 20 February 2023).
- Vasara, E.; Marakis, G.; Breda, J.; Skepastianos, P.; Hassapidou, M.; Kafatos, A.; Rodopaios, N.; Koulouri, A.A.; Cappucio, F.P. Sodium and Potassium Intake in Healthy Adults in Thessaloniki Greater Metropolitan Area—The Salt Intake in Northern Greece (SING) Study. Nutrients 2017, 9, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, H.E.; Johansson, L.; Eggen, A.E.; Johansen, H.; Holvik, K. Sodium and potassium intake assessed by spot and 24-h urine in the population-based TromsØ study 201-2016. Nutrients 2019, 11, 1619. [Google Scholar] [CrossRef] [Green Version]
- Trieu, K.; Ospanova, F.; Tazhibayev, S.; Jewell, J.; Breda, J.; Santos, J.A.; Webster, J. Sodium and potassium intakes in the Kazakhstan population estimated using 24-h urinary excretion: Evidence for national action. Eur. J. Nutr. 2021, 60, 1537–1546. [Google Scholar] [CrossRef]
- Moliterno, P.; Álvarez-Vaz, R.; Pécora, M.; Luzardo, L.; Borgarello, L.; Olascoaga, A.; Marino, C.; Noboa, O.; Staessen, J.A.; Boggia, J. Blood pressure in relation to 23-hour urinary sodium and potassium excretion in a Uruguayan population sample. Int. J. Hypertens. 2018, 2018, 6956078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.; Yu, P.; Yuan, Y.; Li, Q.; Lin, F.; Gao, Z.; Chen, F.; Zhu, P. The relationship between sodium excretion and blood pressure, urine albumin, central retinal arteriolar equivalent. BMC Cardiovasc. Disord. 2016, 16, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durán-Cabral, M.; Fernández-Jalao, I.; Estévez-Santiago, R.; Olmedilla-Alonso, B. Assessment of individual carotenoid and vitamin A dietary intake in overweight and obese Dominican subjects. Nutr. Hosp. 2017, 34, 407–415. [Google Scholar] [CrossRef]
- Del Rosario, P. El Consumo de Alimentos en República Dominicana; Instituto Dominicano de Investigaciones Agropecuarias y Forestales (IDIAF): Santo Domingo, DO, USA, 2021. [Google Scholar]
- Johnson, C.; Mohan, S.; Rogers, K.; Shivashankar, R.; Thout, S.R.; Gupta, P.; He, F.J.; MacGregor, G.A.; Webster, J.; Krishnan, A.; et al. Mean dietary salt intake in urban andrural areas in India: A population survey of 1395 persons. J. Am. Heart Assoc. 2017, 6, e004547. [Google Scholar] [CrossRef]
- McLean, R.M. Measuring population sodium intake: A review of methods. Nutrients 2014, 6, 4651–4662. [Google Scholar] [CrossRef] [Green Version]
- Mannheimer, B.; Sterea-Grossu, A.; Falhammar, H.; Calissendorff, J.; Skov, J.; Lindh, J.D. Current and future burdens of heat-related hyponatremia: A nationwide register-based study. J. Clin. Endocrinol. Metab. 2022, 107, e2388–e2393. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies); Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H. Scientific opinion on dietary reference values for potassium. EFSA J. 2016, 14, 459. [Google Scholar]
- Huang, L.; Wang, H.; Wang, Z.; Wang, Y.; Zhang, B.; Ding, G. Associations of dietary sodium, potassium, and sodium to potassium ratio with blood pressure–regional disparities in China. Nutrients 2020, 12, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, P.; Pérez, W. Situación del Consumo de sal y Sodio en Centroamérica y República Dominicana. INCAP. Notas Técnicas PP/NT/079. 2018. Available online: http://www.incap.int/index.php/es/publicaciones-destacadas/218-situacion-consumo-sal-sodio-2018 (accessed on 20 February 2023).
- WHO. Salt Reduction. Key Facts. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/salt-reduction (accessed on 20 February 2023).
- AHA, American Heart Association. How Potassium can help Control High Blood Pressure. Available online: https://www.heart.org/en/health-topics/high-blood-pressure/changes-you-can-make-to-manage-high-blood-pressure/how-potassium-can-help-control-high-blood-pressure (accessed on 20 February 2023).



| Age (Years) | 44.5 ± 14.6 (18–80) |
|---|---|
| Sex | Men 78/women 85 |
| Race | Mix-race (mulatto) 125/Black 21/White 15/Asian 2 |
| Diabetes | No 143 (87.7%)/yes 20 (12.3%) |
| Normotensive/Hypertensive | 88 [56 mulatto, 16 black, 3 white]/75 [69 mulatto, 4 black, 13 white/2 Asian] |
| Total Sample (n = 163) 78 M, 85 W | Normotensive (n = 88) 43 M, 45 W | Hypertensive (n = 75) 35 M, 40 W | p Value | 18–45 Years (n = 84) (Normotensive = 64, M = 37, W = 27) (Hypertensive = 20, M = 11, W = 9) | 46–80 Years (n = 79) (Normotensive = 24, M = 6, W = 18) (Hypertensive = 55, M = 24, W = 31) | p Value | |
|---|---|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | 126.2 ± 21.1 [123.3] | 116.3 ± 11.8 [115.8] | 138.0 ± 23.4 [135] | <0.001 | 119.3 ± 15.5 [120.2] | 133.7 ± 23.6 [128.6] | <0.001 |
| Diastolic blood pressure (mm Hg) | 81.2 ± 11.2 [81.0] | 76.6 ± 7.3 [76.6] | 86.7 ± 12.5 [87.3] | <0.001 | 78.7 ± 10.6 [77.6] | 84.0 ± 11.2 [83.6] | 0.001 |
| Heart rate (bpm) | 71.3 ± 10.2 [71.6] | 71.0 ± 9.8 [70.8] | 71.5 ± 10.8 [72.0] | 0.826 | 72.2 ±10.7 [73.0] | 70.2 ± 9.7 [69.3] | 0.077 |
| Anthropometric measurementes | |||||||
| Height (cm) | 166.3 ± 10.0 [165.5] | 167.8 ± 10.6 [166.2] | 164.6 ± 9.1 [164.1] | 0.072 | 169.2 ± 10.3 [169.0] | 163.2 ± 8.8 [162.0] | <0.001 |
| Weight (kg) | 80.6 ± 16.0 [79.7] | 79.0 ± 17.6 [77.8] | 82.4 ± 13.9 [83.1] | 0.054 | 81.8 ± 18.1 [79.1] | 79.4 ± 13.4 [80.0] | 0.717 |
| BMI (kg/m2) | 29.1 ± 4.8 [28.6] | 27.9 ± 4.7 [27.1] | 30.5 ± 4.6 [30.4] | <0.001 | 28.4 ± 4.8 [27.4] | 29.9 ± 4.7 [29.6] | 0.031 |
| Waist (cm) | 95.9 ± 12.3 [95.0] | 92.7 ± 12.0 [92.8] | 99.5 ± 11.7 [99.0] | <0.001 | 93.0 ± 12.2 [92.8] | 98.9 ± 11.8 [98.0] | <0.001 |
| Hip (cm) | 107.5 ± 9.0 [107.0] | 106.3 ± 9.4 [104.0] | 108.9 ± 8.5 [109.0] | 0.015 | 106.5 ± 9.2 [104.2] | 108.4 ± 8.8 [108.5] | 0.081 |
| Waist—hip ratio | 0.89 ± 0.08 [0.89] | 0.87 ± 0.07 [0.87] | 0.91 ± 0.07 [0.92] | <0.001 | 0.87 ± 0.07 [0.88] | 0.91 ± 0.08 [0.91] | 0.002 |
| Body fat (%) | 36.3 ± 9.8 [35.4] | 34.8 ± 9.6 [33.3] | 38.1 ± 9.8 [40.3] | 0.019 | 34.1 ± 9.7 [31.5] | 38.7 ± 9.4 [40.0] | 0.002 |
| sdVisceral fat (%) | 10.6 ± 4.2 [10.0] | 9.2 ± 3.8 [9.0] | 12.1 ± 4.0 [11.5] | <0.001 | 9.3 ± 4.0 [9.0] | 11.8 ± 3.9 [11.0] | <0.001 |
| Muscle mass (%) | 28.5 ± 5.9 [28.0] | 29.4 ± 6.4 [28.8] | 27.4 ± 5.1 [27.6] | 0.038 | 30.0 ± 6.2 [30.9] | 26.9 ± 5.3 [25.9] | <0.001 |
| Urine (24 h) | |||||||
| Volume (mL) | 1775.2 ± 819.1 [1740.0] | 1709.4 ± 842.7 [1680] | 1833.4 ± 777.2 [1780] | 0.257 | 1732.0 ± 839.2 [1575.0] | 1821.0 ± 800.0 [1850] | 0.367 |
| Creatinine (mmol) | 12.4 ± 5.3 [11.5] | 13.3 ± 5.3 [11.5] | 11.5 ± 3.5 [11.5] | 0.493 | 14.8 ± 8.3 [14.1] | 12.0 ± 3.6 [11.2] | <0.001 |
| Endogenus creatinine clearance (ECC) (mL/min) | 108.7 ±35.7 [105.3] | 113.5 ± 37.1 [115.3] | 103.4 ± 33.4 [101.9] | 0.089 | 118.0 ±35.8 [120.7] | 98.8 ± 33.0 [97.1] | <0.001 |
| ECC corrected by surface area (ECC-c) (mL/min) | 100.9 ± 30.6 [102.3] | 105.4 ± 30.1 [105.6] | 96.3 ± 30.3 [96.9] | 0.041 | 107.7 ± 30.5 [109.3] | 93.6 ± 29.2 [93.1] | <0.001 |
| Sodium (mmol) | 99.4 ± 46.5 [89.0] | 100.8 ± 47.3 [96.5] | 97.9 ± 46.1 [84.5] | 0.670 | 105.4 ± 46.8 [98.5] | 92.9 ± 45.5 [81.0] | 0.058 |
| Potassium (mmol) | 35.0 ± 17.5 [31.0] | 37.8 ± 20.2 [33.2] | 31.8 ± 13.1 [29.0] | 0.123 | 35.2 ± 17.6 [31.0] | 34.8 ± 17.5 [31.2] | 0.976 |
| Sodium: potassium ratio 1 | 3.1 ± 1.3 [2.9] | 3.0 ± 1.3 [2.9] | 3.2 ± 1.3 [3.0] | 0.255 | 3.3 ± 1.3 [3.1] | 2.9 ± 1.1 [2.9] | 0.040 |
| Na: creatinine ratio 2 | 9.1 ± 6.1 [7.4] | 9.4 ± 7.0 [6.8] | 8.8 ± 4.8 [7.8] | 0.562 | 9.1 ± 6.7 [6.4] | 9.1 ± 5.4 [8.0] | 0.273 |
| K: creatinine ratio 2 | 3.2 ± 2.3 [2.7] | 3.5 ± 2.8 [2.7] | 2.9 ± 1.5 [2.6] | 0.067 | 3.0 ±2.3 [2.4] | 3.5 ± 2.4 [3.0] | 0.013 |
| Microalbumin (mg) | 10.4 ± 27.9 [4.8] | 7.0 ± 16.2 [4.3] | 14.4 ± 37.0 [5.5] | 0.005 | 11.0 ±33.8 [4.5] | 9.7 ± 20.0 [5.2] | 0.616 |
| Blood | |||||||
| Cholesterol (mg/dayL) | 193.0 ± 47.4 [189.7] | 194.8 ± 48.0 [190.5] | 190.6 ± 47.3 [189.6] | 0.687 | 188.4 ± 44.5 [185.2] | 198.3 ± 50.3 [198.7] | 0.258 |
| HDL-cholesterol (mg/dayL) | 48.8 ± 14.6 [46.5] | 51.4 ± 13.3 [49.1] | 45.9 ± 15.7 [42.8] | 0.002 | 50.6 ± 12.7 [48.7] | 47.2 ± 16.5 [44.6] | 0.051 |
| LDL-chol.(mg/dayL) | 122.2 ± 42.6 [42.6] | 123.4 ± 43.9 [122.5] | 120.7 ± 41.3 [125.5] | 0.844 | 118.3 ± 42.8 [121.0] | 126.7 ± 42.3 [127.0] | 0.338 |
| VLDL-cholesterol (mg/dayL) | 21.9 ± 11.1 [19.5] | 20.2 ± 10.8 [17.9] | 24.0 ± 11.2 [20.6] | 0.006 | 19.6 ± 11.2 [14.6] | 24.5 ± 10.5 [22.3] | <0.001 |
| Triglycerides (mg/dayL) | 111.6 ± 60.6 [97.3] | 101.0 ± 54.2 [89.3] | 120.1 ± 55.8 [103.0] | 0.004 | 98.0 ± 56.2 [73.2] | 122.5 ± 52.7 [111.3] | <0.001 |
| Creatinine (mg/dayL) | 0.92 ± 0.28 [0.87] | 0.88 ± 0.22 [0.85] | 0.96 ± 0.34 [0.92] | 0.137 | 0.94 ± 0.29 [0.93] | 0.90 ± 0.28 [0.85] | 0.267 |
| Glucaemia (mg/dayL) | 103.5 ± 31.1 [97.1] | 97.5 ± 25.1 [95.8] | 110.5 ± 35.9 [99.4] | 0.010 | 95.1 ± 14.5 [93.3] | 112.4 ± 40.4 [100.4] | 0.001 |
| Total Group (n = 163) | 18–45 Years (n = 84) | 46–80 Years (n = 79) | Normotensive (n = 88) | Hypertensive (n = 75) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | SBP | DBP | |
| Waist-hip index | 0.432 (<0.001) | 0.259 (<0.001) | 0.508 (<0.001) | 0.316 (0.003) | 0.249 (0.027) | 0.121 (0.286) | 0.497 (<0.001) | 0.235 (0.027) | 0.219 (0.060) | 0.149 (0.203) |
| BMI | 0.367 (<0.001) | 0.261 (<0.001) | 0.374 (<0.001) | 0.326 (0.002) | 0.264 (0.019) | 0.097 (0.395) | 0.407 (<0.001) | 0.262 (0.014) | 0.112 (0.337) | 0.006 (0.956) |
| Body fat | 0.067 (0.394) | 0.196 (0.013) | −0.183 (0.095) | 0.197 (0.072) | 0.124 (0.279) | 0.028 (0.807) | −0.120 (0.264) | 0.203 (0.058) | 0.028 (0.816) | 0.011 (0.924) |
| Muscle mass | 0.013 (0.867) | −0.137 (0.082) | 0.333 (0.002) | −0.127 (0.250) | −0.143 (0.211) | −0.016 (0.899) | 0.247 (0.021) | −0.072 (0.507) | −0.018 (0.876) | −0.046 (0.698) |
| Visceral fat | 0.492 (<0.001) | 0.293 (<0.001) | 0.559 (<0.001) | 0.318 (0.003) | 0.294 (0.009) | 0.157 (0.169) | 0.544 (<0.001) | 0.234 (0.029) | 0.221 (0.058) | 0.083 (0.481) |
| Sodium | 0.078 (0.323) | 0.009 (0.910) | 0.024 (0.827) | −0.031 (0.778) | 0.194 (0.086) | 0.111 (0.331) | 0.125 (0.245) | −0.139 (0.196) | 0.095 (0.418) | 0.204 (0.080) |
| Potassium | 0.022 (0.783) | −0.102 (0.193) | 0.099 (0.370) | −0.037 (0.740) | −0.053 (0.643) | −0.141 (0.216) | 0.249 (0.019) | −0.002 (0.986) | −0.063 (0.589) | −0.111 (0.344) |
| Sodium-to-potassium | 0.093 (0.237) | 0.153 (0.052) | −0.063 (0.567) | 0.030 (0.788) | 0.350 (0.002) | 0.373 (<0.001) | −0.094 (0.383) | −0.114 (0.291) | 0.215 (0.064) | 0.395 (<0.001) |
| Sodium-to-creatinine | 0.039 (0.618) | 0.082 (0.299) | −0.187 (0.088) | 0.024 (0.826) | 0.241 (0.032) | 0.142 (0.213) | −0.134 (0.214) | −0.055 (0.608) | 0.244 (0.035) | 0.268 (0.020) |
| Potassium-to-creatinine | −0.063 (0.427) | −0.063 (0.426) | −0.170 (0.123) | −0.036 (0.743) | −0.059 (0.604) | −0.160 (0.158) | −0.086 (0.428) | −0.020 (0.856) | 0.046 (0.692) | −0.057 (0.629) |
| Creatinine 24 h-urine | 0.116 (0.141) | −0.053 (0.505) | 0.429 (<0.001) | 0.040 (0.721) | −0.046 (0.688) | −0.035 (0.763) | 0.415 (<0.001) | −0.007 (0.951) | −0.185 (0.113) | −0.106 (0.366) |
| Microalbumin | 0.219 (0.005) | 0.187 (0.017) | 0.262 (0.016) | 0.269 (0.013) | 0.156 (0.169) | 0.084 (0.460) | 0.171 (0.111) | 0.086 (0.426) | 0.089 (0.450) | 0.142 (0.224) |
| Creatinine (serum) | 0.326 (<0.001) | 0.045 (0.565) | 0.507 (<0.001) | 0.051 (0.646) | 0.239 (0.034) | 0.127 (0.264) | 0.430 (<0.001) | 0.047 (0.663) | 0.180 (0.123) | −0.025 (0.829) |
| CCE-c | −0.195 (0.013) | −0.185 (0.018) | −0.087 (0.429) | −0.149 (0.175) | −0.218 (0.054) | −0.146 (0.198) | −0.006 (0.956) | −0.174 (0.104) | −0.334 (0.003) | −0.142 (0.223) |
| Systolic Blood Pressure | |||||
| beta | S.E. | p | 95% CI | ||
| Total sample | Constant | 96.7 | 9.9 | 0.000 | 77.3, 116.1 |
| Age | 0.6 | 0.10 | <0.001 | 0.4, 0.8 | |
| Sex (man) | 11.2 | 3.5 | 0.001 | 4.4, 18.1 | |
| Sex (woman) | 0 | ||||
| BMI—normoweight | −12.3 | 4.0 | 0.002 | −20.2, 4.4 | |
| BMI—overweight | −8–3 | 3.2 | 0.009 | −14.5, 2.1 | |
| BMI—obese | 0 | ||||
| 18–45 years | Constant | 114.4 | 4.8 | 0.000 | 105.0, 123.8 |
| Sex (man) | 14.02 | 2.87 | 0.001 | 8.40, 19.64 | |
| Sex (woman) | 0 | ||||
| BMI—normoweight | −12.07 | 3.72 | 0.001 | −19.35, −4.79 | |
| BMI—overweight | −10.7 | 3.2 | 0.001 | −16.95, −4.49 | |
| BMI—obese | 0 | ||||
| 46–80 years | Constant | 118.6 | 7.7 | 0.000 | 103.6, 133.6 |
| Sodium-to-potassium ratio | 6.7 | 2.4 | 0.005 | 2.07, 11.34 | |
| Diastolic blood pressure | |||||
| beta | S.E. | p | 95% IC | ||
| Total sample | Constant | 70.8 | 5.8 | 0.000 | 59.5, 82.1 |
| Age | 0.18 | 0.06 | 0.002 | 0.06, 0.29 | |
| BMI—normoweight | −6.9 | 2.4 | 0.003 | −11.5, −2.3 | |
| BMI—overweight | −2.1 | 1.9 | 0.268 | −5.7, 1.6 | |
| BMI—obese | 0 | ||||
| 18–45 years | Constant | 78.3 | 3.7 | 0.000 | 71.1, 85.5 |
| BMI—normoweight | −9.44 | 2.87 | 0.001 | − 3.8, 10.8 | |
| BMI—overweight | −5.1 | 2.5 | 0.039 | −9.9, −0.26 | |
| BMI—obese | 0 | ||||
| 46–80 years | Constant | 73.4 | 3.6 | 0.000 | 66.4, 80.4 |
| Sodium-to-potassium ratio | 3.8 | 1.1 | <0.001 | 1.6, 5.9 | |
| Sodium Intake (g/Day) | Salt Intake (g/Day) | Potassium Intake (g/Day) | Dietary Na-to-K (mmol) * | |
|---|---|---|---|---|
| Total sample (n = 163) | 2.29 ± 1.07 [2.05] | 5.8 ± 2.7 [5.2] | 1.37 ± 0.68 [1.21] | 2.9 |
| Normotensive (n = 88) | 2.32 ± 1.09 [2.22] | 5.9 ± 2.8 [5.6] | 1.47 ± 0.79 [1.30] a | 2.9 |
| Hypertensive (n = 75) | 2.25 ± 1.06 [1.94] | 5.7 ± 2.7 [4.9] | 1.24 ± 0.51 [1.17] | 3.0 |
| Aged 18–45 years | ||||
| Normotensive (n = 64) | 2.45 ± 1.12 [2.36] | 6.2 ± 2.9 [6.0] | 1.43 ± 0.73 [1.24] | 3.1 |
| Hypertensive (n = 20) | 2.32 ± 0.94 [2.16] | 5.9 ± 2.4 [5.5] | 1.19 ± 0.52 [1.03] | 3.0 |
| Aged 46–80 years | ||||
| Normotensive (n = 24) | 1.95 ± 0.92 [1.85] | 5.0 ± 2.3 [4.7] | 1.60 ± 0.94 [1.36] b | 2.3 |
| Hypertensive (n = 55) | 2.22 ± 1.10 [1.91] | 5.6 ± 2.8 [4.8] | 1.26 ± 0.52 [1.20] | 3.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Lithgow, C.H.; Durán-Cabral, M.; Winter-Matos, A.; García-Estrella, K.; García-Durán, J.; Di-Sanzo, E.; Martínez-De-La-Cruz, N.; Rodríguez-Abreu, J.; Olmedilla-Alonso, B. Assessment of 24 h Sodium and Potassium Urinary Excretion in Normotensive and Hypertensive Dominican Adults. Nutrients 2023, 15, 3197. https://doi.org/10.3390/nu15143197
García-Lithgow CH, Durán-Cabral M, Winter-Matos A, García-Estrella K, García-Durán J, Di-Sanzo E, Martínez-De-La-Cruz N, Rodríguez-Abreu J, Olmedilla-Alonso B. Assessment of 24 h Sodium and Potassium Urinary Excretion in Normotensive and Hypertensive Dominican Adults. Nutrients. 2023; 15(14):3197. https://doi.org/10.3390/nu15143197
Chicago/Turabian StyleGarcía-Lithgow, Carlos Heriberto, Madeline Durán-Cabral, Alexandra Winter-Matos, Kilsaris García-Estrella, Julen García-Durán, Estefanía Di-Sanzo, Nicole Martínez-De-La-Cruz, Julia Rodríguez-Abreu, and Begoña Olmedilla-Alonso. 2023. "Assessment of 24 h Sodium and Potassium Urinary Excretion in Normotensive and Hypertensive Dominican Adults" Nutrients 15, no. 14: 3197. https://doi.org/10.3390/nu15143197
APA StyleGarcía-Lithgow, C. H., Durán-Cabral, M., Winter-Matos, A., García-Estrella, K., García-Durán, J., Di-Sanzo, E., Martínez-De-La-Cruz, N., Rodríguez-Abreu, J., & Olmedilla-Alonso, B. (2023). Assessment of 24 h Sodium and Potassium Urinary Excretion in Normotensive and Hypertensive Dominican Adults. Nutrients, 15(14), 3197. https://doi.org/10.3390/nu15143197