β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers—A Systematic Review of Clinical Trials
Abstract
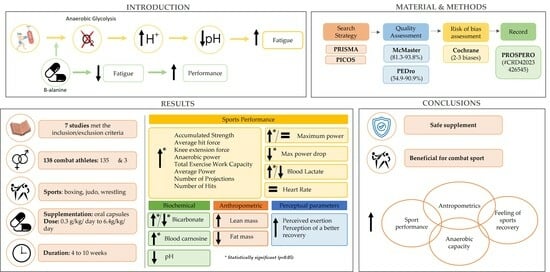
:1. Introduction
2. Materials and Methods
2.1. Search Methods
2.2. Elegibility Criteria
2.3. Methodological Quality and Risk-of-Bias Assessment
2.4. Data Extraction
3. Results
3.1. Study Selection
3.2. Quality Assessment
3.3. Risk-of-Bias Assessment
3.4. Characteristics of the Participants and Interventions
3.5. Outcome Assessment
3.5.1. Sport Performance
- Strength
- Power
- Total exercise work capacity
- Heart rate
- Vertical Jump
- Combat-specific parameters
3.5.2. Perception Parameters
3.5.3. Anthropometric Parameters
3.5.4. Biochemical Biomarkers
- Serum Carnosine
- Bicarbonate (HCO3)
- pH
- Blood Lactate
3.5.5. Adverse Effects
4. Discussion
4.1. β-Alanine Supplementation
4.2. Sports Performance
4.3. Anthropometric Parameters
4.4. Perception Parameters
4.5. Biochemical Biomarkers
4.6. Adverse Effects
4.7. Limitations
4.8. Strengths
5. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Scopus
- Web of Science
- Pubmed
References
- Barley, O.R.; Chapman, D.W.; Guppy, S.N.; Abbiss, C.R. Considerations When Assessing Endurance in Combat Sport Athletes. Front. Physiol. 2019, 10, 205. [Google Scholar] [CrossRef]
- Chaabène, H.; Hachana, Y.; Franchini, E.; Mkaouer, B.; Chamari, K. Physical and physiological profile of elite karate athletes. Sports Med. 2012, 42, 829–843. [Google Scholar] [PubMed]
- Coelho-E-Silva, M.J.; Sousa-E-Silva, P.; Morato, V.S.; Costa, D.C.; Martinho, D.V.; Rama, L.M.; Valente-Dos-Santos, J.; Werneck, A.O.; Tavares, O.M.; Conde, J.; et al. Allometric Modeling of Wingate Test among Adult Male Athletes from Combat Sports. Medicina 2020, 56, 480. [Google Scholar] [CrossRef] [PubMed]
- Ruddock, A.; James, L.; French, D.; Rogerson, D.; Driller, M.; Hembrough, D. High-Intensity Conditioning for Combat Athletes: Practical Recommendations. Appl. Sci. 2021, 11, 10658. [Google Scholar] [CrossRef]
- James, L.P.; Haff, G.G.; Kelly, V.G.; Beckman, E.M. Towards a Determination of the Physiological Characteristics Distinguishing Successful Mixed Martial Arts Athletes: A Systematic Review of Combat Sport Literature. Sports Med. 2016, 46, 1525–1551. [Google Scholar] [CrossRef] [PubMed]
- Atakan, M.M.; Li, Y.; Koşar, Ş.N.; Turnagöl, H.H.; Yan, X. Evidence-Based Effects of High-Intensity Interval Training on Exercise Capacity and Health: A Review with Historical Perspective. Int. J. Environ. Res. Public. Health. 2021, 18, 7201. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Allen, D.G.; Lännergren, J. Muscle fatigue: Lactic acid or inorganic phosphate the major cause? News Physiol. Sci. 2002, 17, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232. [Google Scholar] [CrossRef]
- Lancha Junior, A.H.; de Salles Painelli, V.; Saunders, B.; Artioli, G.G. Nutritional Strategies to Modulate Intracellular and Extracellular Buffering Capacity During High-Intensity Exercise. Sports Med. 2015, 45, S71–S81. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Del Valle Soto, M.; Adams, D.P.; Gutiérrez-Abejón, E.; Seco-Calvo, J. Impact of Optimal Timing of Intake of Multi-Ingredient Performance Supplements on Sports Performance, Muscular Damage, and Hormonal Behavior across a Ten-Week Training Camp in Elite Cyclists: A Randomized Clinical Trial. Nutrients 2021, 13, 3746. [Google Scholar] [CrossRef] [PubMed]
- Hobson, R.M.; Saunders, B.; Ball, G.; Harris, R.C.; Sale, C. Effects of β-alanine supplementation on exercise performance: A meta-analysis. Amino Acids. 2012, 43, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Tallon, M.J.; Dunnett, M.; Boobis, L.; Coakley, J.; Kim, H.J.; Fallowfield, J.L.; Hill, C.A.; Sale, C.; Wise, J.A. The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids. 2006, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet. 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.T.; Smith-Ryan, A.E.; Stout, J.R.; Hoffman, J.R.; Wilborn, C.D.; Sale, C.; Kreider, R.B.; Jäger, R.; Earnest, C.P.; Bannock, L.; et al. International society of sports nutrition position stand: Beta-Alanine. J. Int. Soc. Sports Nutr. 2015, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Varanoske, A.; Stout, J.R. Effects of β-Alanine Supplementation on Carnosine Elevation and Physiological Performance. Adv. Food Nutr. Res. 2018, 84, 183–206. [Google Scholar] [PubMed]
- Church, D.D.; Hoffman, J.R.; Varanoske, A.N.; Wang, R.; Baker, K.M.; La Monica, M.B.; Beyer, K.S.; Dodd, S.J.; Oliveira, L.P.; Harris, R.C.; et al. Comparison of Two β-Alanine Dosing Protocols on Muscle Carnosine Elevations. J. Am. Coll. Nutr. 2017, 36, 608–616. [Google Scholar] [CrossRef]
- Naderi, A.; de Oliveira, E.P.; Ziegenfuss, T.N.; Willems, M.E.T. Timing, optimal dose and intake duration of dietary supplements with evidence-based use in sports nutrition. J. Exerc. Nutr. Biochem. 2016, 20, 1–12. [Google Scholar] [CrossRef]
- Sports Nutrition Market Size, Share & Trends Analysis Report by Product Type (Sports Drink, Sports Supplements, Sports Food), by Distribution Channel (E-commerce, Brick and Mortar), by Region, and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/sports-nutrition-market (accessed on 24 August 2023).
- Fernández-Lázaro, D.; Seco-Calvo, J.; Pascual-Fernández, J.; Domínguez-Ortega, C.; Del Valle Soto, M.; Mielgo-Ayuso, J. 6-Week Supplementation with Tribulus terrestris L. to Trained Male CrossFit® Athletes on Muscle, Inflammation, and Antioxidant Biomarkers: A Randomized, Single-Blind, Placebo-Controlled Trial. Int. J. Environ. Res. Public. Health 2022, 19, 16158. [Google Scholar] [CrossRef] [PubMed]
- International Olympic Committee. Tokyo 2020 Summer Olympics—Athletes, Medals and Results. Available online: https://olympics.com/es/olympic-games/tokyo-2020 (accessed on 9 August 2023).
- Straus, S.E.; Glasziou, P.; Richardson, W.S.H.R. Evidence-Based Medicine: How to Practice and Teach It, 4th ed.; Churchill Livingstone Elsevier: Edinburgh, UK; Available online: https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-ebm/ (accessed on 31 March 2023).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Stewart, C.; Pollock, N.; Letts, L.; Bosch, J.; Westmorland, M. Guidelines for Critical Review of Qualitative Studies; McMaster University Occupational Therapy Evidence-Based Practice Research Group: Hamilton, ON, Canada, 1998; pp. 1–9. [Google Scholar]
- Moseley, A.M.; Elkins, M.R.; Van der Wees, P.J.; Pinheiro, M.B. Using research to guide practice: The Physiotherapy Evidence Database (PEDro). Braz. J. Phys. Ther. 2020, 24, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, K.; Rashidlamir, A.; Dokht, E.H. The Effect of 4 Weeks of Strength Training and Beta-Alanine Supplementation on Anaerobic Power and Carnosine Level in Boxer Players. J. Sci. Sport. Exerc. 2023, 5, 62–69. [Google Scholar] [CrossRef]
- de Andrade Kratz, C.; de Salles Painelli, V.; de Andrade Nemezio, K.M.; da Silva, R.P.; Franchini, E.; Zagatto, A.M.; Gualano, B.; Artioli, G.G. Beta-alanine supplementation enhances judo-related performance in highly-trained athletes. J. Sci. Med. Sport. 2017, 20, 403–408. [Google Scholar] [CrossRef]
- Donovan, T.; Ballam, T.; Morton, J.P.; Close, G.L. β-alanine improves punch force and frequency in amateur boxers during a simulated contest. Int. J. Sport. Nutr. Exerc. Metab. 2012, 22, 331–337. [Google Scholar] [CrossRef]
- Halz, M.; Kaszuba, M.; Helbin, J.; Krzysztofik, S.; Suchanecka, A.; Zając, A. Beta-alanine supplementation and anaerobic performance in highly trained judo athletes. Balt. J. Health Phys. Act. 2022, 14, 1. [Google Scholar] [CrossRef]
- Kern, B.D.; Robinson, T.L. Effects of β-alanine supplementation on performance and body composition in collegiate wrestlers and football players. J. Strength Cond. Res. 2011, 25, 1804–1815. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Song, H.S.; Yoon, D.H.; Fukuda, D.H.; Kim, S.H.; Park, D.H. The effects of 10 weeks of β-alanine supplementation on peak power, power drop, and lactate response in Korean national team boxers. J. Exerc. Rehabil. 2018, 14, 985–992. [Google Scholar] [CrossRef]
- Lopez-Grueso, R.; Marco, A.A.; Marín, J.M.S.; Carretero, C.M. Beta-alanine supplementation seems to increase physical performance and acute recovery in competitive judokas. Eur. J. Hum. Mov. 2014, 33, 123–136. [Google Scholar]
- World Anti-Doping Agency. Prohibited List. Available online: https://www.wada-ama.org/sites/default/files/resources/files/2022list_final_en.pdf (accessed on 10 August 2023).
- Sport Integrity Australia (Australian Gov). Supplements in Sport. Available online: https://www.sportintegrity.gov.au/what-we-do/anti-doping/supplements-sport (accessed on 10 August 2023).
- Tamaki, N.; Tsunemori, F.; Wakabayashi, M.; Hama, T. Effect of histidine-free and -excess diets on anserine and carnosine contents in rat gastrocnemius muscle. J. Nutr. Sci. Vitaminol. 1977, 23, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Blancquaert, L.; Everaert, I.; Bex, T.; Taes, Y.; Calders, P.; Achten, E.; Derave, W. Meal and beta-alanine coingestion enhances muscle carnosine loading. Med. Sci. Sports Exerc. 2013, 45, 1478–1485. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.A.; Ajit, S.K.; Kaftan, E.J.; Pausch, M.H. MrgD activation inhibits KCNQ/M-currents and contributes to enhanced neuronal excitability. J. Neurosci. 2007, 27, 4492–4496. [Google Scholar] [CrossRef] [PubMed]
- Décombaz, J.; Beaumont, M.; Vuichoud, J.; Bouisset, F.; Stellingwerff, T. Effect of slow-release β-alanine tablets on absorption kinetics and paresthesia. Amino Acids. 2012, 43, 67–76. [Google Scholar] [CrossRef]
- Woodward, M.; Debold, E.P. Acidosis and phosphate directly reduce myosin’s force-generating capacity through distinct molecular mechanisms. Front. Physiol. 2018, 10, 377831. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lázaro, D.; Domínguez-Ortega, C.; Busto, N.; Santamaría-Peláez, M.; Roche, E.; Gutiérez-Abejón, E.; Mielgo-Ayuso, J. Influence of N-Acetylcysteine Supplementation on Physical Performance and Laboratory Biomarkers in Adult Males: A Systematic Review of Controlled Trials. Nutrients 2023, 15, 2463. [Google Scholar] [CrossRef]
- Blancquaert, L.; Everaert, I.; Derave, W. Beta-alanine supplementation, muscle carnosine and exercise performance. Curr. Opin. Clin. Nutr. Metab. Care. 2015, 18, 63–70. [Google Scholar] [CrossRef]
- Artioli, G.G.; Gualano, B.; Smith, A.; Stout, J.; Lancha, A.H. Role of β-alanine supplementation on muscle carnosine and exercise performance. Med. Sci. Sports Exerc. 2010, 42, 1162–1173. [Google Scholar] [CrossRef]
- Saunders, B.; Elliott-Sale, K.; Artioli, G.G.; Swinton, P.A.; Dolan, E.; Roschel, H.; Sale, C.; Gualano, B. β-alanine supplementation to improve exercise capacity and performance: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 658–669. [Google Scholar] [CrossRef]
- Derave, W.; Ozdemir, M.S.; Harris, R.C.; Pottier, A.; Reyngoudt, H.; Koppo, K.; Wise, J.A.; Achten, E. Beta-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters. J. Appl. Physiol. 2007, 103, 1736–1743. [Google Scholar] [CrossRef] [PubMed]
- Ducker, K.J.; Dawson, B.; Wallman, K.E. Effect of beta-alanine supplementation on 800-m running performance. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Sas-Nowosielski, K.; Wyciślik, J.; Kaczka, P. Beta-Alanine Supplementation and Sport Climbing Performance. Int. J. Environ. Res. Public. Health. 2021, 18, 5370. [Google Scholar] [CrossRef] [PubMed]
- Kendrick, I.P.; Harris, R.C.; Kim, H.J.; Kim, C.K.; Dang, V.H.; Lam, T.Q.; Bui, T.T.; Smith, M.; Wise, J.A. The effects of 10 weeks of resistance training combined with beta-alanine supplementation on whole body strength, force production, muscular endurance and body composition. Amino Acids. 2008, 34, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.A.; Ross, R.; Kang, J.; Magrelli, J.; Neese, K.; Faigenbaum, A.D.; Wise, J.A. Beta-alanine and the hormonal response to exercise. Int. J. Sports Med. 2008, 29, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Hipkiss, A.R.; Michaelis, J.; Syrris, P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995, 371, 81–85. [Google Scholar] [CrossRef] [PubMed]
- James, R.M.; Cooper, S.B.; Robertson, J.; Martin, D.; Harris, R.C.; Sale, C. Effect of β-alanine supplementation on 20 km cycling time trial performance. Rev. Bras. Educ. Fís. Esporte. 2014, 28, 395–403. [Google Scholar] [CrossRef]
- Hobson, R.M.; Harris, R.C.; Martin, D.; Smith, P.; Macklin, B.; Gualano, B.; Sale, C. Effect of beta-alanine, with and without sodium bicarbonate, on 2000-m rowing performance. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Portington, K.J.; Pascoe, D.D.; Webster, M.J.; Anderson, L.H.; Rutland, R.R.; Gladden, L.B. Effect of induced alkalosis on exhaustive leg press performance. Med. Sci. Sports Exerc. 1998, 30, 523–528. [Google Scholar] [CrossRef]
- Van Thienen, R.; Van Proeyen, K.; Eynde, B.V.; Puype, J.; Lefere, T.; Hespel, P. Beta-alanine improves sprint performance in endurance cycling. Med. Sci. Sports Exerc. 2009, 41, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, K.M.; Wright, G.A.; Glenn Brice, A.; Doberstein, S.T. The effect of beta-alanine supplementation on power performance during repeated sprint activity. J. Strength Cond. Res. 2010, 24, 79–87. [Google Scholar] [CrossRef]
- Mendez-Villanueva, A.; Edge, J.; Suriano, R.; Hamer, P.; Bishop, D. The Recovery of Repeated-Sprint Exercise Is Associated with PCr Resynthesis, while Muscle pH and EMG Amplitude Remain Depressed. PLoS ONE 2012, 7, 0051977. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.A.; Smith, A.E.; Kendall, K.L.; Stout, J.R.; Cramer, J.T. Six weeks of high-intensity interval training with and without beta-alanine supplementation for improving cardiovascular fitness in women. J. Strength Cond. Res. 2010, 24, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.E.; Walter, A.A.; Graef, J.L.; Kendall, K.L.; Moon, J.R.; Lockwood, C.M.; Fukuda, D.H.; Beck, T.W.; Cramer, J.T.; Stout, J.R. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. J. Int. Soc. Sports Nutr. 2009, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport. Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Januszko, P.; Lange, E. Nutrition, supplementation and weight reduction in combat sports: A review. AIMS Public Health 2021, 8, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Cramer, J.T.; Zoeller, R.F.; Torok, D.; Costa, P.; Hoffman, J.R.; Harris, R.C.; O’Kroy, J. Effects of beta-alanine supplementation on the onset of neuromuscular fatigue and ventilatory threshold in women. Amino Acids. 2007, 32, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Faigenbaum, A.D.; Ross, R.; Kang, J.; Stout, J.R.; Wise, J.A. Short-duration beta-alanine supplementation increases training volume and reduces subjective feelings of fatigue in college football players. Nutr. Res. 2008, 28, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Rascon, J.; Trujillo, E.; Morales-Acuña, F.; Gurovivh, A.N. Differences between Males and Females in Determining Exercise Intensity. Int. J. Exerc. Sci. 2020, 13, 1305. [Google Scholar]
- Robergs, R.A.; Ghiasvand, F.; Parker, D. Biochemistry of exercise-induced metabolic acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R502–R516. [Google Scholar] [CrossRef] [PubMed]
- Perim, P.; Marticorena, F.M.; Ribeiro, F.; Barreto, G.; Gobbi, N.; Kerksick, C.; Dolan, E.; Saunders, B. Can the Skeletal Muscle Carnosine Response to Beta-Alanine Supplementation Be Optimized? Front. Nutr. 2019, 6, 135. [Google Scholar] [CrossRef]


| Study, Year | Item | Total | % | Quality Score | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | ||||
| Alabsi et al., 2022 [28] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| De Andrade et al., 2017 [29] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Donovan et al., 2012 [30] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 15 | 93.8 | E |
| Halz et al., 2022 [31] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Kern et al., 2011 [32] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 14 | 87.5 | VG |
| Kim et al., 2018 [33] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 13 | 81.3 | VG |
| López-Grueso et al., 2014 [34] | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 15 | 93.8 | E |
| Study, Year | Items | Total | % | Quality Score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||||
| Alabsi et al., 2022 [28] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| De Andrade et al., 2017 [29] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 | 90.9 | E |
| Donovan et al., 2012 [30] | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 7 | 63.64 | G |
| Halz et al., 2022 [31] | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| Kern et al., 2011 [32] | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 | 81.82 | E |
| Kim et al., 2018 [33] | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 | 72.7 | G |
| López-Grueso et al., 2014 [34] | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 6 | 54.5 | G |
| Study, Year | Items | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Alabsi et al., 2022 [28] |  |  |  |  |  |  |  |  | 6 |
| De Andrade et al., 2017 [29] |  |  |  |  |  |  |  |  | 6 |
| Donovan et al., 2012 [30] |  |  |  |  |  |  |  |  | 5 |
| Halz et al., 2022 [31] |  |  |  |  |  |  |  |  | 6 |
| Kern et al., 2011 [32] |  |  |  |  |  |  |  |  | 6 |
| Kim et al., 2018 [33] |  |  |  |  |  |  |  |  | 6 |
| López-Grueso et al., 2014 [34] |  |  |  |  |  |  |  |  | 5 |
| Characteristics | Types | Study |
|---|---|---|
| Level of participants | Amateur athletes | [28,30] |
| Competition athletes | [29,31,32] | |
| Elite athletes | [33,34] | |
| Pharmaceutical form | Oral supplementation by capsules | [28,29,30,31,32,33,34] |
| Dosages used | 0.3 g/kg/ day | [28] |
| 4 g/day | [32] | |
| 4.9 g/day or 5.4 g/day | [33] | |
| 6 g/day | [30,34] | |
| 6.4 g/day | [29] | |
| 4 g/day/2 weeks + 6 g/day/2 weeks | [31] | |
| Divided dose in the day | 2 times a day | [32] |
| 3 times a day | [31,33,34] | |
| 4 times a day | [29,30] | |
| Unspecified | [28] | |
| Dose schedule | With the main meals | [29,31,34] |
| Immediately after main meals | [33] | |
| Breakfast and lunch | [32] | |
| Unspecified | [28,30] | |
| Duration (weeks) | 4 | [28,29,30,31,34] |
| 8 | [32] | |
| 10 | [33] |
| First Author, Year of Publication, and Country | Study Design | Participants (Baseline Sample Size, Age, Sex, Withdrawals, and Final Group Sample Size) | Intervention | Outcomes | Results |
|---|---|---|---|---|---|
| Alabsi et al. [28], 2022, Iran | Randomized, double-blind crossover, placebo-controlled trial | 18 ♂ well-trained Korean boxers BA = n = 9 Age (mean ± SD) 24.44 ± 5.76 years Height (mean ± SD) 78.66 ± 3.31 cm Body mass (mean ± SD) 80.95 ± 13.74 kg BMI (mean ± SD) 22.88 ± 3.33 kg/m2 Fat mass (mean ± SD) 23.01 ± 3.20%. PLA: n = 9 Age (mean ± SD) 22.00 ± 4.69 years Height (mean ± SD) 173.77 ± 4.26 cm Body mass (mean ± SD) 69.13 ± 10.75 kg BMI (mean ± SD) 25.3 0 ± 3.72 kg/m2 Fat mass (mean ± SD) 15.14 ± 6.99%. Study withdrawals: 0 | 0.3 g/kg of BA or PLA (maltodextrin) Encapsulated in 800 mg capsules Supplementation time: 4 weeks | MaxP AP MPD CAR in blood LAC in blood | BA vs. PLA ↔ MaxP ↔ AP ↓ MPD ↑* CAR in blood ↔ LAC in blood BA vs. Pre-Supple ↔ MaxP ↑* AP ↓* MPD ↑* CAR in blood ↑* LAC in blood |
| de Andrade et al. [29], 2017, Brazil | Randomized, double-blind crossover, placebo-controlled trial | 23 ♂ judo athletes BA: n = 12 Age (mean ± SD) 17 ± 2 years Body mass (mean ± SD) 74.2 0 ± 11.60 kg Experience (mean ± SD) 9 ± 3 years PLA: n = 11 Age (mean ± SD) 19 ± 3 years Body mass (mean ± SD) 71.5 0 ± 10.70 kg Experience (mean ± SD) 11 ± 4 years Study withdrawals: 0 | 6.4 g/day of BA or PLA (dextrose) Encapsulated in 800 mg capsules (4 times daily) Supplementation time: 4 weeks | P x C TP Blood pH LAC in blood HCO3 in blood | BA vs. PLA ↑* P x C ↑* TP ↔ Blood pH ↔ LAC in blood ↔ HCO3 in blood BA vs. Pre-Supple ↑* P x C ↑* TP ↓ Blood pH ↑* LAC in blood ↓* HCO3 in blood |
| Donovan et al. [30], 2012, United Kingdom | Randomized, controlled, single-blind trial | 16 ♂ boxing competitors BA: n = 8, PLA: n = 8 Age (mean ± SD) 25 ± 4 years Height (mean ± SD) 1.74 ± 0.07 m Body mass (mean ± SD) 78.4 0 ± 7.60 kg 25 ± 4 years, 78.4 0 ± 7.60 kg, 1.74 ± 0.07 m Study withdrawals: 0 | 6 g/day of encapsulated BA or PLA (maltodextrin) divided into 4 doses per day (1.5 g) Supplementation time: 4 weeks | HR LAC in blood MedF TH AS | BA vs. PLA ↔ HR ↑* LAC in blood ↑* MedF ↑* TH ↑* AS BA vs. Pre-Supple ↔ HR ↑* LAC in blood ↑* MedF ↑* TH ↑* AS |
| Halz et al. [31], 2022, Poland | Randomized, double-blind crossover, placebo-controlled trial | 16 ♂ elite judo athletes BA: n = 8 Age (mean ± SD) 20.7 0 ± 3.20 years Height (mean ± SD) 177.2 0 ± 2.60 cm Body mass (mean ± SD) 81.5 0 ± 3.90 kg VO2max (mean ± SD) 54.5 0 ± 3.80 mL/kg/min Fat mass (mean ± SD) 10.90 ± 2.60% PLA: n= 8 Age (mean ± SD) 22.1 0 ± 2.80 years Height (mean ± SD) 178.30 ± 4.90 cm Body mass (mean ± SD) 78.40 ± 5.10 kg VO2max (mean ± SD) 52.60 ± 4.90 mL/kg/min Fat mass (mean ± SD) 9.80 ± 3.20% Study withdrawals: 0 | 4 g/day of BA or PLA for 2 weeks divided into 3 intakes 6 g/day of BA or PLA for 2 weeks divided into 3 intakes Supplementation time: 4 weeks | TLW TUW AVP lower AVP higher LAC in blood HCO3 in blood | BA vs. PLA ↑* TLW ↑* TUW ↔ AVP lower ↑* AVP superior ↑* LAC in blood ↑* HCO3 in blood BA vs. Pre-Supple ↑* TLW ↑* TUW ↔ AVP lower ↑* AVP superior ↑* LAC in blood ↑* HCO3 in blood |
| Kern et al. [32], 2011, USA | Randomized, double-blind crossover, placebo-controlled trial | 37 ♂ wrestling and football competitors W BA: n = 10 Age (mean ± SD) 20.10 ± 2.06 years Height (mean ± SD) 174.0 0 ± 8.07 cm Body mass (mean ± SD) 73.8 0 ± 15.64 lbs W PLA: n = 12 Age (mean ± SD) 19.8 0 ± 1.83 years Height (mean ± SD) 174.80 ± 6.55 cm Body mass (mean ± SD) 77.60 ± 13.84 lbs Body mass (mean ± SD) Study withdrawals: 0 | Dosage: 4 g/day of BA or PLA (dextrose) Encapsulated divided into 2 doses per day Supplementation time: 8 weeks | AP LAC LM FM | W BA vs. W PLA ↑* AP ↓ LAC ↑ LM ↓ FM W BA vs. W Pre-Supple ↑* AP ↓ LAC ↑ LM ↓ FM |
| Kim et al. [33], 2018, Korea | Double-blind crossover study | 20 ♂ Korean boxing athletes BA: n = 10 Age (mean ± SD) 23.00 ± 1.82 years Height (mean ± SD) 180.41 ± 7.42 cm Body mass (mean ± SD) 77.25 ± 20.64 kg Fat mass (mean ± SD) 12.30 ± 7.89% BMI (mean ± SD) 23.6 ± 5.51 kg/m2 Experience (mean ± SD) 7.27 ± 0.95 years PLA: n = 10 Age (mean ± SD) 22.2 0 ± 2.21 years Height (mean ± SD) 178.59 ± 6.33 cm Body mass (mean ± SD) 75.31 ± 19.21 kg Fat mass (mean ± SD) 13.87 ± 6.44% BMI (mean ± SD) 24.03 ± 4.49 kg/m2 Experience (mean ± SD) 7.41 ± 0.73 years Study withdrawals: 1 (injury) BA n = 9 | 4.9 g/day of BA or PLA in capsules for 49–69 kg. 5.4 g/day of BA or PLA in capsules for 75–91 kg. In 3/ times a day (18–30 mg/kg/meal) Supplementation time: 10 weeks | MaxP lower MPD upper EF left knee VJ LAC in blood | BA vs. PLA ↑* MaxP upper ↓* MPD upper ↑ EF left knee ↑ VJ ↔ LAC in blood BA vs. Pre-Supple ↑ MaxP lower ↔ MPD upper ↑* EF left knee ↑* VJ ↑* LAC in blood |
| López-Grueso et al. [34], 2014, Spain | Quasi-experimental, single-blind trial | 8 judokas of the Spanish judo team BA: n = 4 (3 ♂, 1 ♀) Age (mean ± SD) 23.50 ± 0.70 years Height (mean ± SD) 1.60 ± 0.04 cm Body mass (mean ± SD) 61.4 0 ± 1.40 kg PLA: n = 4 (2 ♂, 2 ♀) Age (mean ± SD) 25.0 0 ± 1.00 years Height (mean ± SD) 1.70 ± 0.04 cm Body mass (mean ± SD) 66.30 ± 9.90 kg Study withdrawals: 3 (injury) BA n = 2 (1 ♂, 1 ♀) PLA n = 3 (1 ♂, 2 ♀) | 6 g/day of BA or PLA (maltodextrin) Encapsulated divided into 3 doses per day Supplementation time: 35 days | TP P PE PBR LAC in blood | BA vs. PLA ↑ TP ↑ P ↑ PE ↑ PBR ↔ LAC in blood BA vs. Pre-Supple ↑ TP ↑ P ↑ PE ↑ PBR ↔ LAC in blood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Fiandor, E.M.; García, J.F.; Busto, N.; Santamaría-Peláez, M.; Gutiérrez-Abejón, E.; Roche, E.; Mielgo-Ayuso, J. β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers—A Systematic Review of Clinical Trials. Nutrients 2023, 15, 3755. https://doi.org/10.3390/nu15173755
Fernández-Lázaro D, Fiandor EM, García JF, Busto N, Santamaría-Peláez M, Gutiérrez-Abejón E, Roche E, Mielgo-Ayuso J. β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers—A Systematic Review of Clinical Trials. Nutrients. 2023; 15(17):3755. https://doi.org/10.3390/nu15173755
Chicago/Turabian StyleFernández-Lázaro, Diego, Emma Marianne Fiandor, Juan F. García, Natalia Busto, Mirian Santamaría-Peláez, Eduardo Gutiérrez-Abejón, Enrique Roche, and Juan Mielgo-Ayuso. 2023. "β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers—A Systematic Review of Clinical Trials" Nutrients 15, no. 17: 3755. https://doi.org/10.3390/nu15173755
APA StyleFernández-Lázaro, D., Fiandor, E. M., García, J. F., Busto, N., Santamaría-Peláez, M., Gutiérrez-Abejón, E., Roche, E., & Mielgo-Ayuso, J. (2023). β-Alanine Supplementation in Combat Sports: Evaluation of Sports Performance, Perception, and Anthropometric Parameters and Biochemical Markers—A Systematic Review of Clinical Trials. Nutrients, 15(17), 3755. https://doi.org/10.3390/nu15173755