Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design of Diet and Pb Exposures
2.2. Hematological Analyses
2.3. Tissue Harvests
2.3.1. Microdissection of Cortical and Hippocampal Brain Tissue
2.3.2. Femur
2.4. Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
2.5. Statistical Analysis
3. Results
3.1. Maternal Iron Deficiency Is Not Exacerbated by Environmentally Relevant Pb Exposures
3.2. Maternal ID Increases Circulating and Stored Maternal Pb Levels
3.3. Maternal ID and Pb Exposure Affect Pup Weights, but Not Litter Size
3.4. Maternal ID Causes an Iron Deficiency Anemia (IDA) in Offspring That Is Not Exacerbated by Pb Exposure
3.5. Maternal ID and Pb Exposure Disrupt Offspring Circulating and Brain Iron Levels in a Region-Specific Manner
3.6. Elevations in Pb Burden Attributed to ID Are Not Always Reflected in BPb Levels
3.7. Maternal ID Contributes to Brain Pb Accumulation in Offspring
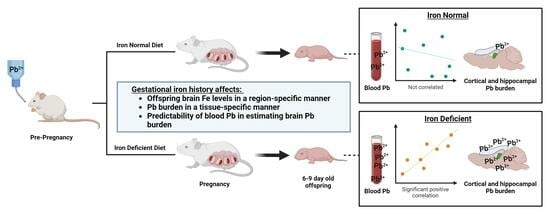
3.8. Blood Pb Levels of Offspring Correlate with Brain Pb Levels in ID but Not in Offspring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. The Global Prevalence of Anaemia in 2011; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Iglesias-Vazquez, L.; Gimeno, M.; Coronel, P.; Caspersen, I.H.; Basora, J.; Arija, V. Maternal factors associated with iron deficiency without anaemia in early pregnancy: ECLIPSES study. Ann. Hematol. 2023, 102, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Munro, M.G. Iron Deficiency in Pregnancy and Postpartum: It Is Time for a Change. Obstet. Gynecol. 2023, 141, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Hua, M.; Shi, D.; Xu, W.; Zhu, L.; Hao, X.; Zhu, B.; Shu, Q.; Lozoff, B.; Geng, F.; Shao, J. Differentiation between fetal and postnatal iron deficiency in altering brain substrates of cognitive control in pre-adolescence. BMC Med. 2023, 21, 167. [Google Scholar] [CrossRef] [PubMed]
- Barks, A.K.; Liu, S.X.; Georgieff, M.K.; Hallstrom, T.C.; Tran, P.V. Early-Life Iron Deficiency Anemia Programs the Hippocampal Epigenomic Landscape. Nutrients 2021, 13, 3857. [Google Scholar] [CrossRef]
- Wiegersma, A.M.; Dalman, C.; Lee, B.K.; Karlsson, H.; Gardner, R.M. Association of Prenatal Maternal Anemia with Neurodevelopmental Disorders. JAMA Psychiatry 2019, 76, 1294–1304. [Google Scholar] [CrossRef]
- Mudd, A.T.; Fil, J.E.; Knight, L.C.; Lam, F.; Liang, Z.P.; Dilger, R.N. Early-Life Iron Deficiency Reduces Brain Iron Content and Alters Brain Tissue Composition Despite Iron Repletion: A Neuroimaging Assessment. Nutrients 2018, 10, 135. [Google Scholar] [CrossRef]
- Skalicky, A.; Meyers, A.F.; Adams, W.G.; Yang, Z.; Cook, J.T.; Frank, D.A. Child food insecurity and iron deficiency anemia in low-income infants and toddlers in the United States. Matern. Child Health J. 2006, 10, 177–185. [Google Scholar] [CrossRef]
- Reid, B.M.; East, P.; Blanco, E.; Doom, J.R.; Burrows, R.A.; Correa-Burrows, P.; Lozoff, B.; Gahagan, S. Early-life adversity is associated with poor iron status in infancy. Dev. Psychopathol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Pirkle, J.L.; Brody, D.J.; Gunter, E.W.; Kramer, R.A.; Paschal, D.C.; Flegal, K.M.; Matte, T.D. The decline in blood lead levels in the United States. The National Health and Nutrition Examination Surveys (NHANES). J. Am. Med. Assoc. 1994, 272, 284–291. [Google Scholar] [CrossRef]
- Muntner, P.; Menke, A.; Desalvo, K.B.; Rabito, F.A.; Batuman, V. Continued Decline in Blood Lead Levels Among Adults in the United States. Arch. Intern. Med. 2005, 165, 2155. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.P.; Sun, Y.; Zheng, Y.X. Trends in Blood Lead Levels in the U.S. From 1999 to 2016. Am. J. Prev. Med. 2021, 60, e179–e187. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.; Yeter, D.; Aschner, M.; Wheeler, D.C. Estimated IQ points and lifetime earnings lost to early childhood blood lead levels in the United States. Sci. Total Environ. 2021, 778, 146307. [Google Scholar] [CrossRef] [PubMed]
- Malcoe, L.H.; Lynch, R.A.; Keger, M.C.; Skaggs, V.J. Lead sources, behaviors, and socioeconomic factors in relation to blood lead of native american and white children: A community-based assessment of a former mining area. Environ. Health Perspect. 2002, 110 (Suppl. S2), 221–231. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B.; Cornwell, C.R.; Courtney, J.G.; Ettinger, A.S. Blood Lead Levels in U.S. Children Ages 1–11 Years, 1976–2016. Environ. Health Perspect. 2021, 129, 37003. [Google Scholar] [CrossRef]
- Marshall, A.T.; Betts, S.; Kan, E.C.; McConnell, R.; Lanphear, B.P.; Sowell, E.R. Association of lead-exposure risk and family income with childhood brain outcomes. Nat. Med. 2020, 26, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Apte, A.; Bradford, K.; Dente, C.; Smith, R.N. Lead toxicity from retained bullet fragments: A systematic review and meta-analysis. J. Trauma Acute Care Surg. 2019, 87, 707–716. [Google Scholar] [CrossRef]
- Olufemi, A.C.; Mji, A.; Mukhola, M.S. Potential Health Risks of Lead Exposure from Early Life through Later Life: Implications for Public Health Education. Int. J. Environ. Res. Public Health 2022, 19, 16006. [Google Scholar] [CrossRef]
- Tasin, F.R.; Ahmed, A.; Halder, D.; Mandal, C. On-going consequences of in utero exposure of Pb: An epigenetic perspective. J. Appl. Toxicol. JAT 2022, 42, 1553–1569. [Google Scholar] [CrossRef]
- Ouyang, L.; Li, Q.; Rao, S.; Su, R.; Zhu, Y.; Du, G.; Xie, J.; Zhou, F.; Feng, C.; Fan, G. Cognitive outcomes caused by low-level lead, cadmium, and mercury mixture exposure at distinct phases of brain development. Food Chem. Toxicol. Int. J. Public Br. Ind. Biol. Res. Assoc. 2023, 175, 113707. [Google Scholar] [CrossRef] [PubMed]
- Bellinger, D.C. Neurological and behavioral consequences of childhood lead exposure. PLoS Med. 2008, 5, e115. [Google Scholar] [CrossRef]
- Rocha, A.; Trujillo, K.A. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. NeuroToxicology 2019, 73, 58–80. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.; Hryhorczuk, D.; Lanphear, B.P.; Rankin, K.M.; Lewis, D.A.; Forst, L.; Rosenberg, D. The impact of low-level lead toxicity on school performance among children in the Chicago Public Schools: A population-based retrospective cohort study. Environ. Health 2015, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Lanphear, B.P.; Hornung, R.; Khoury, J.; Yolton, K.; Baghurst, P.; Bellinger, D.C.; Canfield, R.L.; Dietrich, K.N.; Bornschein, R.; Greene, T.; et al. Erratum: “Low-Level Environmental Lead Exposure and Children’s Intellectual Function: An International Pooled Analysis”. Environ. Health Perspect. 2019, 127, 99001. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of Childhood Blood Lead Levels with Cognitive Function and Socioeconomic Status at Age 38 Years and with IQ Change and Socioeconomic Mobility between Childhood and Adulthood. J. Am. Med. Assoc. 2017, 317, 1244. [Google Scholar] [CrossRef] [PubMed]
- Buka, I.; Hervouet-Zeiber, C. Lead toxicity with a new focus: Addressing low-level lead exposure in Canadian children. Paediatr. Child Health 2019, 24, 293–294. [Google Scholar] [CrossRef] [PubMed]
- Barry, P.S. Concentrations of lead in the tissues of children. Occup. Environ. Med. 1981, 38, 61–71. [Google Scholar] [CrossRef]
- Gulson, B.; Taylor, A.; Eisman, J. Bone remodeling during pregnancy and post-partum assessed by metal lead levels and isotopic concentrations. Bone 2016, 89, 40–51. [Google Scholar] [CrossRef]
- Tellez-Rojo, M.M.; Hernandez-Avila, M.; Lamadrid-Figueroa, H.; Smith, D.; Hernandez-Cadena, L.; Mercado, A.; Aro, A.; Schwartz, J.; Hu, H. Impact of bone lead and bone resorption on plasma and whole blood lead levels during pregnancy. Am. J. Epidemiol. 2004, 160, 668–678. [Google Scholar] [CrossRef]
- Ettinger, A.S.; Hu, H.; Hernandez-Avila, M. Dietary calcium supplementation to lower blood lead levels in pregnancy and lactation. J. Nutr. Biochem. 2007, 18, 172–178. [Google Scholar] [CrossRef]
- Lamadrid-Figueroa, H.; Tellez-Rojo, M.M.; Hernandez-Cadena, L.; Mercado-Garcia, A.; Smith, D.; Solano-Gonzalez, M.; Hernandez-Avila, M.; Hu, H. Biological markers of fetal lead exposure at each stage of pregnancy. J. Toxicol. Environ. Health Part A 2006, 69, 1781–1796. [Google Scholar] [CrossRef]
- Stojsavljevic, A.; Perovic, M.; Nesic, A.; Mikovic, Z.; Manojlovic, D. Levels of non-essential trace metals and their impact on placental health: A review. Environ. Sci. Pollut. Res. Int. 2022, 29, 43662–43674. [Google Scholar] [CrossRef] [PubMed]
- Bressler, J.P.; Olivi, L.; Cheong, J.H.; Kim, Y.; Bannon, D. Divalent Metal Transporter 1 in Lead and Cadmium Transport. Ann. N. Y. Acad. Sci. 2004, 1012, 142–152. [Google Scholar] [CrossRef]
- Bannon, D.I.; Abounader, R.; Lees, P.S.; Bressler, J.P. Effect of DMT1 knockdown on iron, cadmium, and lead uptake in Caco-2 cells. Am. J. Physiol. Cell Physiol. 2003, 284, C44–C50. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.; Zaher, M.; Abd El-Hafez, M.; Morsy, A.; Saleh, R. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res. Notes 2010, 3, 133. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.O.; Tsaih, S.W.; Schwartz, J.; Wright, R.J.; Hu, H. Association between iron deficiency and blood lead level in a longitudinal analysis of children followed in an urban primary care clinic. J. Pediatr. 2003, 142, 9–14. [Google Scholar] [CrossRef]
- Schell, L.M.; Denham, M.; Stark, A.D.; Ravenscroft, J.; Parsons, P.; Schulte, E. Relationship between blood lead concentration and dietary intakes of infants from 3 to 12 months of age. Environ. Res. 2004, 96, 264–273. [Google Scholar] [CrossRef]
- Lozoff, B.; Jimenez, E.; Smith, J.B. Double burden of iron deficiency in infancy and low socioeconomic status: A longitudinal analysis of cognitive test scores to age 19 years. Arch. Pediatr. Adolesc. Med. 2006, 160, 1108–1113. [Google Scholar] [CrossRef]
- Kordas, K. Iron, Lead, and Children’s Behavior and Cognition. Annu. Rev. Nutr. 2010, 30, 123–148. [Google Scholar] [CrossRef]
- Shah-Kulkarni, S.; Ha, M.; Kim, B.M.; Kim, E.; Hong, Y.C.; Park, H.; Kim, Y.; Kim, B.N.; Chang, N.; Oh, S.Y.; et al. Neurodevelopment in Early Childhood Affected by Prenatal Lead Exposure and Iron Intake. Medicine 2016, 95, e2508. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Mansouri, B.; Binkowski, L.J.; Blaszczyk, M.; Pirsaheb, M.; Azadi, N.A.; Sloboda, M.; Amirabadizadeh, A.; Javadmoosavi, S.Y. Blood lead concentrations in children with iron deficiency anemia: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2022, 29, 3199–3212. [Google Scholar] [CrossRef]
- Kordas, K.; Burganowski, R.; Roy, A.; Peregalli, F.; Baccino, V.; Barcia, E.; Mangieri, S.; Ocampo, V.; Manay, N.; Martinez, G.; et al. Nutritional status and diet as predictors of children’s lead concentrations in blood and urine. Environ. Int. 2018, 111, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chang, J.Y.; Hong, J.; Shin, S.; Park, J.S.; Oh, S. Low-Level Toxic Metal Exposure in Healthy Weaning-Age Infants: Association with Growth, Dietary Intake, and Iron Deficiency. Int. J. Environ. Res. Public Health 2017, 14, 388. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Chambial, S.; Agrawal, N.; Gothwal, M.; Kathuria, P.; Singh, P.; Sharma, P.; Sharma, P.P. Blood lead levels in antenatal women and its association with iron deficiency anemia and adverse pregnancy outcomes. J. Fam. Med. Prim. Care 2020, 9, 3106–3111. [Google Scholar] [CrossRef]
- Arshad, S.; Arif, A.; Wattoo, J.I. Response of Iron Deficiency Markers to Blood Lead Levels and Synergistic Outcomes at Prenatal Stage. Dose-Response 2022, 20, 155932582211017. [Google Scholar] [CrossRef]
- Davies, S.; Briand, V.; Accrombessi, M.; Fievet, N.; Le Bot, B.; Durand, S.; Agbota, G.; Yovo, E.; Vianou, B.; Sossou, D.; et al. Pre-conception serum ferritin concentrations are associated with metal concentrations in blood during pregnancy: A cohort study in Benin. Environ. Res. 2021, 202, 111629. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Childhood Lead Poisoning Prevention: Populations at Higher Risk; National Center for Environmental Health, Division of Environmental Health Science and Practice: Washington, DC, USA, 2021.
- Ruckart, P.Z.; Jones, R.L.; Courtney, J.G.; LeBlanc, T.T.; Jackson, W.; Karwowski, M.P.; Cheng, P.Y.; Allwood, P.; Svendsen, E.R.; Breysse, P.N. Update of the Blood Lead Reference Value—United States, 2021. MMWR. Morb. Mortal. Wkly. Rep. 2021, 70, 1509–1512. [Google Scholar] [CrossRef]
- Perng, W.; Tamayo-Ortiz, M.; Tang, L.; Sanchez, B.N.; Cantoral, A.; Meeker, J.D.; Dolinoy, D.C.; Roberts, E.F.; Martinez-Mier, E.A.; Lamadrid-Figueroa, H.; et al. Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) Project. BMJ Open 2019, 9, e030427. [Google Scholar] [CrossRef]
- Leasure, J.L.; Giddabasappa, A.; Chaney, S.; Johnson, J.E., Jr.; Pothakos, K.; Lau, Y.S.; Fox, D.A. Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 2008, 116, 355–361. [Google Scholar] [CrossRef]
- Zhao, Z.-H.; Zheng, G.; Wang, T.; Du, K.-J.; Han, X.; Luo, W.-J.; Shen, X.-F.; Chen, J.-Y. Low-level Gestational Lead Exposure Alters Dendritic Spine Plasticity in the Hippocampus and Reduces Learning and Memory in Rats. Sci. Rep. 2018, 8, 3533. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Perera, F.; Jankowski, J.; Rauh, V.; Flak, E.; Caldwell, K.L.; Jones, R.L.; Pac, A.; Lisowska-Miszczyk, I. Prenatal low-level lead exposure and developmental delay of infants at age 6 months (Krakow inner city study). Int. J. Hyg. Environ. Health 2008, 211, 345–351. [Google Scholar] [CrossRef]
- Nigg, J.T.; Knottnerus, G.M.; Martel, M.M.; Nikolas, M.; Cavanagh, K.; Karmaus, W.; Rappley, M.D. Low blood lead levels associated with clinically diagnosed attention-deficit/hyperactivity disorder and mediated by weak cognitive control. Biol. Psychiatry 2008, 63, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.; Liu, Y.; Buchner, V.; Tchounwou, P.B. Neurotoxic effects and biomarkers of lead exposure: A review. Rev. Environ. Health 2009, 24, 15–45. [Google Scholar] [CrossRef] [PubMed]
- Greminger, A. Characterizing the Neurodevelopmental Sequelae in a Dual Insult Model of Gestational Iron Deficiency and Lead (Pb) Exposure. Ph.D. Thesis, University of Rochester, Rochester, NY, USA, 2014. [Google Scholar]
- Brunette, K.E.; Tran, P.V.; Wobken, J.D.; Carlson, E.S.; Georgieff, M.K. Gestational and neonatal iron deficiency alters apical dendrite structure of CA1 pyramidal neurons in adult rat hippocampus. Dev. Neurosci. 2010, 32, 238–248. [Google Scholar] [CrossRef]
- Callahan, L.S.; Thibert, K.A.; Wobken, J.D.; Georgieff, M.K. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev. Neurosci. 2013, 35, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Stansfield, K.H.; Ruby, K.N.; Soares, B.D.; McGlothan, J.L.; Liu, X.; Guilarte, T.R. Early-life lead exposure recapitulates the selective loss of parvalbumin-positive GABAergic interneurons and subcortical dopamine system hyperactivity present in schizophrenia. Transl. Psychiatry 2015, 5, e522. [Google Scholar] [CrossRef]
- Lien, Y.C.; Condon, D.E.; Georgieff, M.K.; Simmons, R.A.; Tran, P.V. Dysregulation of Neuronal Genes by Fetal-Neonatal Iron Deficiency Anemia Is Associated with Altered DNA Methylation in the Rat Hippocampus. Nutrients 2019, 11, 1191. [Google Scholar] [CrossRef]
- Schachtschneider, K.M.; Liu, Y.; Rund, L.A.; Madsen, O.; Johnson, R.W.; Groenen, M.A.; Schook, L.B. Impact of neonatal iron deficiency on hippocampal DNA methylation and gene transcription in a porcine biomedical model of cognitive development. BMC Genom. 2016, 17, 856. [Google Scholar] [CrossRef]
- Rudy, M.; Mayer-Proschel, M. Iron Deficiency Affects Seizure Susceptibility in a Time- and Sex-Specific Manner. ASN Neuro 2017, 9, 1759091417746521. [Google Scholar] [CrossRef]
- Jain, N.B.; Laden, F.; Guller, U.; Shankar, A.; Kazani, S.; Garshick, E. Relation between Blood Lead Levels and Childhood Anemia in India. Am. J. Epidemiol. 2005, 161, 968–973. [Google Scholar] [CrossRef]
- Queirolo, E.I.; Ettinger, A.S.; Stoltzfus, R.J.; Kordas, K. Association of anemia, child and family characteristics with elevated blood lead concentrations in preschool children from Montevideo, Uruguay. Arch. Environ. Occup. Health 2010, 65, 94–100. [Google Scholar] [CrossRef]
- Weston, H.I.; Weston, D.D.; Allen, J.L.; Cory-Slechta, D.A. Sex-dependent impacts of low-level lead exposure and prenatal stress on impulsive choice behavior and associated biochemical and neurochemical manifestations. NeuroToxicology 2014, 44, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.S.; Anderson, D.W.; Kidd, S.K.; Sobolewski, M.; Cory-Slechta, D.A. Sex-dependent effects of lead and prenatal stress on post-translational histone modifications in frontal cortex and hippocampus in the early postnatal brain. NeuroToxicology 2016, 54, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Greminger, A.R.; Mayer-Proschel, M. Identifying the threshold of iron deficiency in the central nervous system of the rat by the auditory brainstem response. ASN Neuro 2015, 7, 1759091415569911. [Google Scholar] [CrossRef] [PubMed]
- Mihaila, C.; Schramm, J.; Strathmann, F.G.; Lee, D.L.; Gelein, R.M.; Luebke, A.E.; Mayer-Pröschel, M. Identifying a Window of Vulnerability during Fetal Development in a Maternal Iron Restriction Model. PLoS ONE 2011, 6, e17483. [Google Scholar] [CrossRef]
- Hubbard, A.C.; Bandyopadhyay, S.; Wojczyk, B.S.; Spitalnik, S.L.; Hod, E.A.; Prestia, K.A. Effect of dietary iron on fetal growth in pregnant mice. Comp. Med. 2013, 63, 127–135. [Google Scholar]
- Zhang, Q.; Lu, X.M.; Zhang, M.; Yang, C.Y.; Lv, S.Y.; Li, S.F.; Zhong, C.Y.; Geng, S.S. Adverse effects of iron deficiency anemia on pregnancy outcome and offspring development and intervention of three iron supplements. Sci. Rep. 2021, 11, 1347. [Google Scholar] [CrossRef]
- Rudy, M.J.; Salois, G.; Cubello, J.; Newell, R.; Mayer-Proschel, M. Gestational iron deficiency affects the ratio between interneuron subtypes in the postnatal cerebral cortex in mice. Development 2023, 150, dev201068. [Google Scholar] [CrossRef]
- Alwan, N.A.; Cade, J.E.; McArdle, H.J.; Greenwood, D.C.; Hayes, H.E.; Simpson, N.A. Maternal iron status in early pregnancy and birth outcomes: Insights from the Baby’s Vascular health and Iron in Pregnancy study. Br. J. Nutr. 2015, 113, 1985–1992. [Google Scholar] [CrossRef]
- Puerto, A.; Trojan, A.; Alvis-Zakzuk, N.R.; Lopez-Saleme, R.; Edna-Estrada, F.; Alvarez, A.; Alvis-Guzman, N.; Zakzuk, J. Iron status in late pregnancy is inversely associated with birth weight in Colombia. Public Health Nutr. 2021, 24, 5090–5100. [Google Scholar] [CrossRef]
- Monk, C.; Georgieff, M.K.; Xu, D.; Hao, X.; Bansal, R.; Gustafsson, H.; Spicer, J.; Peterson, B.S. Maternal Prenatal Iron Status and Tissue Organization in the Neonatal Brain. Pediatr. Res. 2015, 79, 482–488. [Google Scholar] [CrossRef]
- Hect, J.L.; Daugherty, A.M.; Hermez, K.M.; Thomason, M.E. Developmental variation in regional brain iron and its relation to cognitive functions in childhood. Dev. Cogn. Neurosci. 2018, 34, 18–26. [Google Scholar] [CrossRef]
- McFarland, M.J.; Hauer, M.E.; Reuben, A. Half of US population exposed to adverse lead levels in early childhood. Proc. Natl. Acad. Sci. USA 2022, 119, e2118631119. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, M.; Varma, G.; Adams, B.; Anderson, D.W.; Schneider, J.S.; Cory-Slechta, D.A. Developmental Lead Exposure and Prenatal Stress Result in Sex-Specific Reprograming of Adult Stress Physiology and Epigenetic Profiles in Brain. Toxicol. Sci. Off. J. Soc. Toxicol. 2018, 163, 478–489. [Google Scholar] [CrossRef]
- Sobolewski, M.; Abston, K.; Conrad, K.; Marvin, E.; Harvey, K.; Susiarjo, M.; Cory-Slechta, D.A. Lineage- and Sex-Dependent Behavioral and Biochemical Transgenerational Consequences of Developmental Exposure to Lead, Prenatal Stress, and Combined Lead and Prenatal Stress in Mice. Environ. Health Perspect. 2020, 128, 27001. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Schneider, J.S. Strain specific effects of low level lead exposure on associative learning and memory in rats. NeuroToxicology 2017, 62, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fu, X.; Zhang, J.; Xu, C.; Hu, Q.; Lin, W. Association between blood lead level during pregnancy and birth weight: A meta-analysis. Am. J. Ind. Med. 2020, 63, 1085–1094. [Google Scholar] [CrossRef]
- Daniali, S.S.; Yazdi, M.; Heidari-Beni, M.; Taheri, E.; Zarean, E.; Goli, P.; Kelishadi, R. Birth Size Outcomes in Relation to Maternal Blood Levels of Some Essential and Toxic Elements. Biol. Trace Elem. Res. 2023, 201, 4–13. [Google Scholar] [CrossRef]
- Zhu, M.; Fitzgerald, E.F.; Gelberg, K.H.; Lin, S.; Druschel, C.M. Maternal low-level lead exposure and fetal growth. Environ. Health Perspect. 2010, 118, 1471–1475. [Google Scholar] [CrossRef]
- Lee, M.S.; Eum, K.D.; Golam, M.; Quamruzzaman, Q.; Kile, M.L.; Mazumdar, M.; Christiani, D.C. Umbilical Cord Blood Metal Mixtures and Birth Size in Bangladeshi Children. Environ. Health Perspect. 2021, 129, 57006. [Google Scholar] [CrossRef]
- Freire, C.; Amaya, E.; Gil, F.; Murcia, M.; Llop, S.; Casas, M.; Vrijheid, M.; Lertxundi, A.; Irizar, A.; Fernandez-Tardon, G.; et al. Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int. J. Hyg. Environ. Health 2019, 222, 468–478. [Google Scholar] [CrossRef]
- Taylor, C.M.; Tilling, K.; Golding, J.; Emond, A.M. Low level lead exposure and pregnancy outcomes in an observational birth cohort study: Dose-response relationships. BMC Res. Notes 2016, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Kumar, D.; Anupurba, S.; Verma, A.; Kumar, A. Effect of maternal iron deficiency anemia on fetal neural development. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 233–239. [Google Scholar] [CrossRef]
- Kohli, U.A.; Rajput, M.; Venkatesan, S. Association of maternal hemoglobin and iron stores with neonatal hemoglobin and iron stores. Med. J. Armed Forces India 2021, 77, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Lou, J.; Rao, R.; Georgieff, M.K.; Kaciroti, N.; Felt, B.T.; Zhao, Z.Y.; Lozoff, B. Maternal serum ferritin concentration is positively associated with newborn iron stores in women with low ferritin status in late pregnancy. J. Nutr. 2012, 142, 2004–2009. [Google Scholar] [CrossRef] [PubMed]
- Getu, S.; Shiferaw, E.; Melku, M. Neonatal Iron: Factors Influencing its Level and Associated Complications—A Review Article. Clin. Lab. 2020, 66, 239–248. [Google Scholar] [CrossRef]
- Erikson, K.M.; Pinero, D.J.; Connor, J.R.; Beard, J.L. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J. Nutr. 1997, 127, 2030–2038. [Google Scholar] [CrossRef]
- Erikson, K.; Pinero, D.; Connor, J.; Beard, J.L. Iron status and distribution of iron in the brain of developing rats. J. Nutr. 1997, 127, 2030–2038. [Google Scholar] [CrossRef]
- Rentschler, G.; Broberg, K.; Lundh, T.; Skerfving, S. Long-term lead elimination from plasma and whole blood after poisoning. Int. Arch. Occup. Environ. Health 2012, 85, 311–316. [Google Scholar] [CrossRef]
- Bergdahl, I.A.; Vahter, M.; Counter, S.A.; Schutz, A.; Buchanan, L.H.; Ortega, F.; Laurell, G.; Skerfving, S. Lead in plasma and whole blood from lead-exposed children. Environ. Res. 1999, 80, 25–33. [Google Scholar] [CrossRef]
- Sakai, T.; Yanagihara, S.; Kunugi, Y.; Ushio, K. Relationships between distribution of lead in erythrocytes in vivo and in vitro and inhibition of ALA-D. Occup. Environ. Med. 1982, 39, 382–387. [Google Scholar] [CrossRef]
- Bergdahl, I.A.; Grubb, A.; Schütz, A.; Desnick, R.J.; Wetmur, J.G.; Sassa, S.; Skerfving, S. Lead Binding to δ-Aminolevulinic Acid Dehydratase (ALAD) in Human Erythrocytes. Pharmacol. Toxicol. 1997, 81, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, F., Jr.; Tanus-Santos, J.E.; Gerlach, R.F.; Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: Advantages, limitations, and future needs. Environ. Health Perspect. 2005, 113, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Shih, R.A.; Hu, H.; Weisskopf, M.G.; Schwartz, B.S. Cumulative lead dose and cognitive function in adults: A review of studies that measured both blood lead and bone lead. Environ. Health Perspect. 2007, 115, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Rosado, J.L.; Lopez, P.; Kordas, K.; Garcia-Vargas, G.; Ronquillo, D.; Alatorre, J.; Stoltzfus, R.J. Iron and/or Zinc Supplementation Did Not Reduce Blood Lead Concentrations in Children in a Randomized, Placebo-Controlled Trial. J. Nutr. 2006, 136, 2378–2383. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guideline: Daily Iron and Folic Acid Supplementation in Pregnant Women. WHO Guidelines Approved by the Guidelines Review Committee; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Pena-Rosas, J.P.; De-Regil, L.M.; Garcia-Casal, M.N.; Dowswell, T. Daily oral iron supplementation during pregnancy. Cochrane Database Syst. Rev. Online 2015, 7, CD004736. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cubello, J.; Peterson, D.R.; Wang, L.; Mayer-Proschel, M. Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development. Nutrients 2023, 15, 4101. https://doi.org/10.3390/nu15194101
Cubello J, Peterson DR, Wang L, Mayer-Proschel M. Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development. Nutrients. 2023; 15(19):4101. https://doi.org/10.3390/nu15194101
Chicago/Turabian StyleCubello, Janine, Derick R. Peterson, Lu Wang, and Margot Mayer-Proschel. 2023. "Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development" Nutrients 15, no. 19: 4101. https://doi.org/10.3390/nu15194101
APA StyleCubello, J., Peterson, D. R., Wang, L., & Mayer-Proschel, M. (2023). Maternal Iron Deficiency and Environmental Lead (Pb) Exposure Alter the Predictive Value of Blood Pb Levels on Brain Pb Burden in the Offspring in a Dietary Mouse Model: An Important Consideration for Cumulative Risk in Development. Nutrients, 15(19), 4101. https://doi.org/10.3390/nu15194101