Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Size Determination
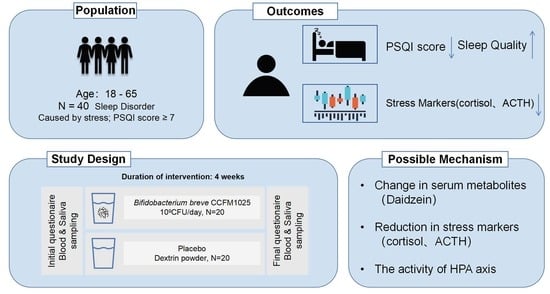
2.3. Study Design
2.4. Sleep Disorder Measurements (Major Outcome Measures)
2.5. Salivary Cortisol Measurements
2.6. Plasma Measurements
2.7. Serum Measurements
2.8. Statistical Analysis
3. Results
3.1. Clinical Grouping and Validation of Insomnia
3.2. CCFM1025 Intervention Enhanced the Sleep Quality of Insomnia Patients
3.3. Effects of CCFM1025 Intervention on Stress Markers in Insomnia Patients
3.4. Metabolomic Analysis Reveals Daidzein as a Biomarker in CCFM1025 Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukherjee, S.; Patel, S.R.; Kales, S.N.; Ayas, N.T.; Strohl, K.P.; Gozal, D.; Malhotra, A. An Official American Thoracic Society Statement: The Importance of Healthy Sleep. Recommendations and Future Priorities. Am. J. Respir. Crit. Care Med. 2015, 191, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Akerstedt, T.; Knutsson, A.; Westerholm, P.; Theorell, T.; Alfredsson, L.; Kecklund, G. Sleep disturbances, work stress and work hours: A cross-sectional study. J. Psychosom. Res. 2002, 53, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Itani, O.; Jike, M.; Watanabe, N.; Kaneita, Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep. Med. 2017, 32, 246–256. [Google Scholar] [CrossRef]
- Van Someren, E.J.W. Brain mechanisms of insomnia: New perspectives on causes and consequences. Physiol. Rev. 2021, 101, 995–1046. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Hong, Z.; Sun, Q.; Dai, Y.; Basta, M.; Tang, X.; Qin, Q. Insomnia symptoms during the early and late stages of the COVID-19 pandemic in China: A systematic review and meta-analysis. Sleep. Med. 2022, 91, 262–272. [Google Scholar] [CrossRef]
- Choi, D.W.; Chun, S.Y.; Lee, S.A.; Han, K.T.; Park, E.C. Association between Sleep Duration and Perceived Stress: Salaried Worker in Circumstances of High Workload. Int. J. Environ. Res. Public. Health 2018, 15, 796. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Sawada, D.; Kawai, T.; Nishida, K.; Kuwano, Y.; Fujiwara, S.; Rokutan, K. Daily intake of Lactobacillus gasseri CP2305 improves mental, physical, and sleep quality among Japanese medical students enrolled in a cadaver dissection course. J. Funct. Foods 2017, 31, 188–197. [Google Scholar] [CrossRef]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-T.; Tsai, Y.-C.; Kuo, T.B.J.; Yang, C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients 2021, 13, 2820. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Bastiaanssen, T.F.S.; Song, L.; Jiang, B.; Zhang, X.; Zhao, J.; Zhang, H.; Chen, W.; Cryan, J.F.; Wang, G. Unraveling the Microbial Mechanisms Underlying the Psychobiotic Potential of a Bifidobacterium breve Strain. Mol. Nutr. Food Res. 2021, 65, 2000704. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Guo, M.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Integrative metabolomic characterization reveals the mediating effect of Bifidobacterium breve on amino acid metabolism in a mouse model of Alzheimer’s disease. Nutrients 2022, 14, 735. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.-k.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Qian, X.; Tian, P.; Lin, G.; Xu, X.; Wang, G.; Zhang, H.; Chen, W. Detection of colonization capacity of probiotic Bifidobacterium breve CCFM1025 in the human gut. Future Microbiol. 2023, 18, 595–606. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Tian, P.; Hou, Y.; Wang, Z.; Jiang, J.; Qian, X.; Qu, Z.; Zhao, J.; Wang, G.; Chen, W. Probiotics Administration Alleviates Cognitive Impairment and Circadian Rhythm Disturbance Induced by Sleep Deprivation. Food Sci. Hum. Wellness 2023. [Google Scholar] [CrossRef]
- Ranjbar, M.; Salehi, A.; Rezaeizadeh, H.; Zarshenas, M.M.; Sadeghniiat-Haghighi, K.; Mirabzadeh, M.; Firoozabadi, A. Efficacy of a Combination of Melissa officinalis L. and Nepeta Menthoides Boiss. & Buhse on Insomnia: A Triple-Blind, Randomized Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2018, 24, 1197–1203. [Google Scholar] [CrossRef]
- Suresh, K. An overview of randomization techniques: An unbiased assessment of outcome in clinical research. J. Hum. Reprod. Sci. 2011, 4, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, J.C.; Gaab, J.; Hellhammer, D.H.; Lintz, D.; Schommer, N.; Kirschbaum, C. Increasing correlations between personality traits and cortisol stress responses obtained by data aggregation. Psychoneuroendocrinology 1997, 22, 615–625. [Google Scholar] [CrossRef]
- Dressle, R.J.; Feige, B.; Spiegelhalder, K.; Schmucker, C.; Benz, F.; Mey, N.C.; Riemann, D. HPA axis activity in patients with chronic insomnia: A systematic review and meta-analysis of case-control studies. Sleep. Med. Rev. 2022, 62, 101588. [Google Scholar] [CrossRef]
- Yuan, M.; Breitkopf, S.B.; Yang, X.; Asara, J.M. A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 2012, 7, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Rodenbeck, A.; Hajak, G. Neuroendocrine dysregulation in primary insomnia. Rev. Neurol. 2001, 157, S57–S61. [Google Scholar]
- Drake, C.L.; Cheng, P.; Almeida, D.M.; Roth, T. Familial Risk for Insomnia Is Associated With Abnormal Cortisol Response to Stress. Sleep 2017, 40, zsx143. [Google Scholar] [CrossRef]
- Holsboer, F.; Ising, M. Stress hormone regulation: Biological role and translation into therapy. Annu. Rev. Psychol. 2010, 61, 81–109. [Google Scholar] [CrossRef]
- Morin, C.M.; Benca, R. Chronic insomnia. Lancet 2012, 379, 1129–1141. [Google Scholar] [CrossRef]
- Levenson, J.C.; Kay, D.B.; Buysse, D.J. The pathophysiology of insomnia. Chest 2015, 147, 1179–1192. [Google Scholar] [CrossRef]
- Johns, M.W.; Gay, T.J.; Masterton, J.P.; Bruce, D.W. Relationship between sleep habits, adrenocortical activity and personality. Psychosom. Med. 1971, 33, 499–508. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Tsigos, C.; Bixler, E.O.; Stratakis, C.A.; Zachman, K.; Kales, A.; Vela-Bueno, A.; Chrousos, G.P. Chronic insomnia and activity of the stress system: A preliminary study. J. Psychosom. Res. 1998, 45, 21–31. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Bixler, E.O.; Lin, H.M.; Prolo, P.; Mastorakos, G.; Vela-Bueno, A.; Kales, A.; Chrousos, G.P. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: Clinical implications. J. Clin. Endocrinol. Metab. 2001, 86, 3787–3794. [Google Scholar] [CrossRef] [PubMed]
- Boehme, M.; Rémond-Derbez, N.; Lerond, C.; Lavalle, L.; Keddani, S.; Steinmann, M.; Rytz, A.; Dalile, B.; Verbeke, K.; Van Oudenhove, L.; et al. Bifidobacterium longum subsp. longum Reduces Perceived Psychological Stress in Healthy Adults: An Exploratory Clinical Trial. Nutrients 2023, 15, 3122. [Google Scholar] [CrossRef] [PubMed]
- Irwin, C.; McCartney, D.; Desbrow, B.; Khalesi, S. Effects of probiotics and paraprobiotics on subjective and objective sleep metrics: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2020, 74, 1536–1549. [Google Scholar] [CrossRef] [PubMed]
- Aras, A.B.; Guven, M.; Akman, T.; Ozkan, A.; Sen, H.M.; Duz, U.; Kalkan, Y.; Silan, C.; Cosar, M. Neuroprotective effects of daidzein on focal cerebral ischemia injury in rats. Neural Regen. Res. 2015, 10, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, D.; Yu, B.; Mao, X.; Huang, Z.; Yu, J.; Luo, J.; Zheng, P.; Luo, Y.; He, J. Effects of Dietary Daidzein Supplementation on Reproductive Performance, Serum Hormones, and Reproductive-Related Genes in Rats. Nutrients 2018, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- Zhihua, H.; Liangdong, L.; Xiao, L.; Fang, C.; Jing, Z. Effect of 3(′)-daidzein sulfonic sodium on the anti-oxidation of retinal ischemia/reperfusion injury in rats. Adv. Exp. Med. Biol. 2010, 664, 585–591. [Google Scholar] [CrossRef]
- Song, X.; Gong, Z.; Liu, K.; Kou, J.; Liu, B.; Liu, K. Baicalin combats glutamate excitotoxicity via protecting glutamine synthetase from ROS-induced 20S proteasomal degradation. Redox Biol. 2020, 34, 101559. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kwon, S.H.; Ma, S.X.; Seo, J.Y.; Lee, B.R.; Kim, K.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. The memory-enhancing effects of 7,8,4′-trihydroxyisoflavone, a major metabolite of daidzein, are associated with activation of the cholinergic system and BDNF signaling pathway in mice. Brain Res. Bull. 2018, 142, 197–206. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kim, S.Y.; Lee, S.Y.; Jang, C.G. 6,7,4′-Trihydroxyisoflavone, a major metabolite of daidzein, improves learning and memory via the cholinergic system and the p-CREB/BDNF signaling pathway in mice. Eur. J. Pharmacol. 2018, 826, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, X.; Zhang, Y.; Zhong, H.; Wang, C.; Gao, P.; Li, B. Daidzein Alleviates Hypothalamic-Pituitary-Adrenal Axis Hyperactivity, Ameliorates Depression-Like Behavior, and Partly Rectifies Circulating Cytokine Imbalance in Two Rodent Models of Depression. Front. Behav. Neurosci. 2021, 15, 671864. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.; Harvey, A.G.; Lockley, S.W.; Dijk, D.-J. Circadian rhythms and disorders of the timing of sleep. Lancet 2022, 400, 1061–1078. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.G.; Resick, P.A.; Yehuda, R. Enhanced cortisol suppression following dexamethasone administration in domestic violence survivors. Am. J. Psychiatry 2005, 162, 1192–1199. [Google Scholar] [CrossRef] [PubMed]





| Variable | Placebo (n = 20) | CCFM1025 (n = 20) | Statistic Value | p Value | |
|---|---|---|---|---|---|
| Age, Mean ± SD | 36.55 ± 11.31 | 38.95 ± 10.59 | 0.693 | 0.493 a | |
| BMI. Mean ± SD | 22.59 ± 3.30 | 22.76 ± 3.05 | 0.168 | 0.866 a | |
| Sex, n (%) | Female | 14 (70) | 12 (60) | 0.440 | 0.507 b |
| Male | 6 (30) | 8 (40) | |||
| Race, n (%) | Chinese, Han nationality | 20 (100) | 20 (100) | N/A | N/A |
| Medication Before inclusion, n (%) | 0 (0) | 0 (0) | N/A | N/A | |
| Educational status, n (%) | Primary | 1 (5) | 0 (0) | 2.534 | 0.469 b |
| Secondary | 3 (15) | 4 (20) | |||
| Higher | 10 (50) | 13 (65) | |||
| Unknown | 6 (30) | 3 (15) | |||
| Alcohol use, n (%) | Non-drinker | 16 (80) | 15 (75) | 0.143 | 0.705 b |
| Occasional drinker | 4 (20) | 5 (25) | |||
| Smokers, n (%) | Non-smoker | 16 (80) | 13 (65) | 1.129 | 0.288 b |
| Smoker | 4 (20) | 7 (35) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Y.; Lu, J.; Qiao, G.; Mao, X.; Zhao, J.; Wang, G.; Tian, P.; Chen, W. Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients 2023, 15, 4700. https://doi.org/10.3390/nu15214700
Lan Y, Lu J, Qiao G, Mao X, Zhao J, Wang G, Tian P, Chen W. Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients. 2023; 15(21):4700. https://doi.org/10.3390/nu15214700
Chicago/Turabian StyleLan, Yuming, Junjie Lu, Guohong Qiao, Xuhua Mao, Jianxin Zhao, Gang Wang, Peijun Tian, and Wei Chen. 2023. "Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial" Nutrients 15, no. 21: 4700. https://doi.org/10.3390/nu15214700
APA StyleLan, Y., Lu, J., Qiao, G., Mao, X., Zhao, J., Wang, G., Tian, P., & Chen, W. (2023). Bifidobacterium breve CCFM1025 Improves Sleep Quality via Regulating the Activity of the HPA Axis: A Randomized Clinical Trial. Nutrients, 15(21), 4700. https://doi.org/10.3390/nu15214700