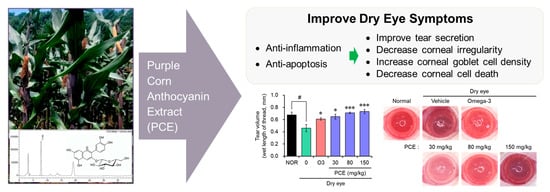
Purple Corn Extract Improves Dry Eye Symptoms in Models Induced by Desiccating Stress and Extraorbital Lacrimal Gland Excision
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Purple Corn Extract
2.2. High-Performance Liquid Chromatography (HPLC) Analysis of Cyanidin-3-O-Glucoside
2.3. Cell Culture
2.4. Cell Viability Assay
2.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Animal Experiments
2.7. Tear Volume Measurement
2.8. Corneal Surface Irregularity
2.9. Periodic Acid–Schiff (PAS) Staining
2.10. Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End Labeling (TUNEL) Staining
2.11. Statistical Analysis
3. Results
3.1. HPLC Analysis of PCE
3.2. PCE Protects Cells against Damage from Desiccation Stress
3.3. PCE Decreases the mRNA Level of Inflammatory Mediators
3.4. PCE Improves Tear Production and Corneal Irregularity in Rats with DED
3.5. PCE Increases Corneal Goblet Cell Density on the Ocular Surface
3.6. PCE Prevents Apoptotic Cell Death in the Corneal Epithelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Galletti, J.G.; de Paiva, C.S. The ocular surface immune system through the eyes of aging. Ocul. Surf. 2021, 20, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Davidson, H.J.; Kuonen, V.J. The tear film and ocular mucins. Vet. Ophthalmol. 2004, 7, 71–77. [Google Scholar] [CrossRef]
- Zhang, X.; M, V.J.; Qu, Y.; He, X.; Ou, S.; Bu, J.; Jia, C.; Wang, J.; Wu, H.; Liu, Z.; et al. Dry Eye Management: Targeting the Ocular Surface Microenvironment. Int. J. Mol. Sci. 2017, 18, 1398. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hindman, H.B. Aging: A predisposition to dry eyes. J. Ophthalmol. 2014, 2014, 781683. [Google Scholar] [CrossRef]
- de Paiva, C.S. Effects of Aging in Dry Eye. Int. Ophthalmol. Clin. 2017, 57, 47–64. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Nichols, K.K. Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape. Ophthalmol. Ther. 2023, 12, 1397–1418. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S. Dry eye syndrome. J. Ophthalmic. Vis. Res. 2011, 6, 192–198. [Google Scholar]
- Rao, S.K.; Mohan, R.; Gokhale, N.; Matalia, H.; Mehta, P. Inflammation and dry eye disease-where are we? Int. J. Ophthalmol. 2022, 15, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, D.; Misra, S.L.; Mugisho, O.O.; Rupenthal, I.D.; Craig, J.P. NLRP3 Inflammasome as a Potential Therapeutic Target in Dry Eye Disease. Int. J. Mol. Sci. 2023, 24, 10866. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Chen, Y.; Chauhan, S.; Dana, R. Ocular surface immunity: Homeostatic mechanisms and their disruption in dry eye disease. Prog. Retin. Eye Res. 2012, 31, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Cristianini, M.; Guillén Sánchez, J.S. Extraction of bioactive compounds from purple corn using emerging technologies: A review. J. Food Sci. 2020, 85, 862–869. [Google Scholar] [CrossRef]
- Colombo, R.; Ferron, L.; Papetti, A. Colored Corn: An Up-Date on Metabolites Extraction, Health Implication, and Potential Use. Molecules 2021, 26, 199. [Google Scholar] [CrossRef]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, B.H.; Jin, B.R.; Park, S.J.; An, H.J. Purple Corn Extract Improves Benign Prostatic Hyperplasia by Regulating Prostate Cell Proliferation and Apoptosis. Agric. Food Chem. 2022, 70, 5561–5569. [Google Scholar] [CrossRef]
- Barabino, S.; Labetoulle, M.; Rolando, M.; Messmer, E.M. Understanding Symptoms and Quality of Life in Patients With Dry Eye Syndrome. Ocul. Surf. 2016, 14, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Kim, H.W.; Won, S.R.; Min, H.K.; Park, K.J.; Park, J.Y.; Ahn, M.S.; Rhee, H.I. Corn husk as a potential source of anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and Identification of Anthocyanins in Corn Cob and Corn Husk from Cacahuacintle Maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef]
- Kang, W.S.; Jung, E.; Kim, J. Aucuba japonica Extract and Aucubin Prevent Desiccating Stress-Induced Corneal Epithelial Cell Injury and Improve Tear Secretion in a Mouse Model of Dry Eye Disease. Molecules 2018, 23, 2599. [Google Scholar] [CrossRef] [PubMed]
- Scarpellini, C.; Ramos Llorca, A.; Lanthier, C.; Klejborowska, G.; Augustyns, K. The Potential Role of Regulated Cell Death in Dry Eye Diseases and Ocular Surface Dysfunction. Int. J. Mol. Sci. 2023, 24, 731. [Google Scholar] [CrossRef] [PubMed]
- Portal, C.; Gouyer, V.; Gottrand, F.; Desseyn, J.L. Ocular mucins in dry eye disease. Exp. Eye Res. 2019, 186, 107724. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Rolando, M.; Benitez Del Castillo, J.M.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Ji, Y.W.; Lee, S.M.; Shim, J.; Noh, H.; Yeo, A.; Park, C.; Park, M.S.; Chang, E.J.; Lee, H.K. Activation of HIF-1α (hypoxia inducible factor-1α) prevents dry eye-induced acinar cell death in the lacrimal gland. Cell Death Dis. 2014, 5, e1309. [Google Scholar] [CrossRef]
- Barabino, S. Is dry eye disease the same in young and old patients? A narrative review of the literature. BMC Ophthalmol. 2022, 22, 85. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, E.A.; Tiffany, J.M.; Yokoi, N.; Bron, A.J. A mass and solute balance model for tear volume and osmolarity in the normal and the dry eye. Prog. Retin. Eye Res. 2010, 29, 59–78. [Google Scholar] [CrossRef]
- Mohamed, H.B.; Abd El-Hamid, B.N.; Fathalla, D.; Fouad, E.A. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur. J. Pharm. Sci. 2022, 175, 106206. [Google Scholar] [CrossRef]
- Cevallos-Casals, B.A.; Cisneros-Zevallos, L. Stoichiometric and kinetic studies of phenolic antioxidants from Andean purple corn and red-fleshed sweetpotato. J. Agric. Food Chem. 2003, 51, 3313–3319. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Giusti, M.M. Characterization of anthocyanin-rich waste from purple corncobs (Zea mays L.) and its application to color milk. J. Agric. Food Chem. 2005, 53, 8775–8781. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Giusti, M.M. Effects of extraction conditions on improving the yield and quality of an anthocyanin-rich purple corn (Zea mays L.) color extract. J. Food Sci. 2007, 72, C363–C368. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Corrales, M.; Zhang, C.; Hu, X.; Ma, Y.; Tauscher, B. Composition and thermal stability of anthocyanins from chinese purple corn (Zea mays L.). J. Agric. Food Chem. 2008, 56, 10761–10766. [Google Scholar] [CrossRef]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and bound phenolic compounds in different Bolivian purple corn (Zea mays L.) cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Zilić, S.; Serpen, A.; Akıllıoğlu, G.; Gökmen, V.; Vančetović, J. Phenolic compounds, carotenoids, anthocyanins, and antioxidant capacity of colored maize (Zea mays L.) kernels. J. Agric. Food Chem. 2012, 60, 1224–1231. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, X.; Guo, L.; Zhang, Z.; Xu, C. Toxicological assessment on safety of maize purple plant pigment. J. Chin. Inst. Food Sci. Technol. 2007, 7, 141–143. [Google Scholar]
- Nabae, K.; Hayashi, S.M.; Kawabe, M.; Ichihara, T.; Hagiwara, A.; Tamano, S.; Tsushima, Y.; Uchida, K.; Koda, T.; Nakamura, M.; et al. A 90-day oral toxicity study of purple corn color, a natural food colorant, in F344 rats. Food Chem. Toxicol. 2008, 46, 774–780. [Google Scholar] [CrossRef]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef]







| Target Genes | Primer Sequences | |
|---|---|---|
| IL-1β | Forward | 5′-GCACGATGCACCTGTACGAT-3′ |
| Reverse | 5′-AGACATCACCAAGCTTTTTTGCT-3′ | |
| IL-6 | Forward | 5′-CAGGAATTGAATGGGTTTGC-3′ |
| Reverse | 5′-AAACCAAGGCACAGTGGAAC-3′ | |
| IL-8 | Forward | 5′-TTTTGCCAAGGAGTGCTAAAGA-3′ |
| Reverse | 5′-AACCCTCTGCACCCAGTTTTC-3′ | |
| IL-12 | Forward | 5′-CTTGTGGCTACCCTGGTCCT-3′ |
| Reverse | 5′-GAGTTTGTCTGGCCTTCTGG-3′ | |
| TNF-α | Forward | 5′-CTGGGCAGGTCTACTTTGGG-3′ |
| Reverse | 5′-CTGGAGGCCCCAGTTTGAAT-3′ | |
| iNOS | Forward | 5′-GGTGGAAGCGGTAACAAAGG-3′ |
| Reverse | 5′-TGCTTGGTGGCGAAGATGA-3′ | |
| COX-2 | Forward | 5′-GCCAAGCACTTTTGGTGGAG-3′ |
| Reverse | 5′-GGGACAGCCCTTCACGTTAT-3′ | |
| GAPDH | Forward | 5′-GACCACAGTCCATGCCATCA-3′ |
| Reverse | 5′-TCCACCACCCTGTTGCTGTA-3′ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Choi, A.; Lee, H.-H.; Park, S.J.; Kim, B.-H. Purple Corn Extract Improves Dry Eye Symptoms in Models Induced by Desiccating Stress and Extraorbital Lacrimal Gland Excision. Nutrients 2023, 15, 5063. https://doi.org/10.3390/nu15245063
Lee J-M, Choi A, Lee H-H, Park SJ, Kim B-H. Purple Corn Extract Improves Dry Eye Symptoms in Models Induced by Desiccating Stress and Extraorbital Lacrimal Gland Excision. Nutrients. 2023; 15(24):5063. https://doi.org/10.3390/nu15245063
Chicago/Turabian StyleLee, Jae-Min, Arin Choi, Hee-Hwan Lee, Sang Jae Park, and Byung-Hak Kim. 2023. "Purple Corn Extract Improves Dry Eye Symptoms in Models Induced by Desiccating Stress and Extraorbital Lacrimal Gland Excision" Nutrients 15, no. 24: 5063. https://doi.org/10.3390/nu15245063
APA StyleLee, J. -M., Choi, A., Lee, H. -H., Park, S. J., & Kim, B. -H. (2023). Purple Corn Extract Improves Dry Eye Symptoms in Models Induced by Desiccating Stress and Extraorbital Lacrimal Gland Excision. Nutrients, 15(24), 5063. https://doi.org/10.3390/nu15245063