Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review
Abstract
:1. Introduction
2. Methods
3. Results
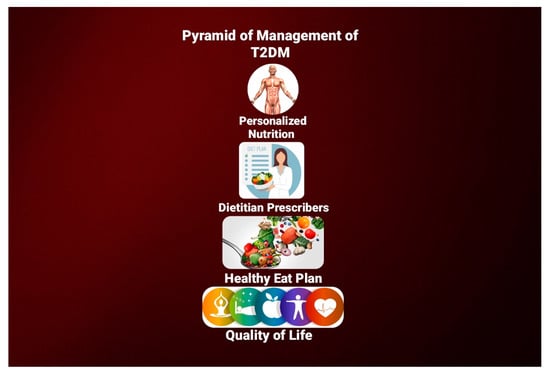
3.1. The Importance of Individuality in Nutritional Management of T2DM
3.2. Dietary Prescription and Macronutrient Distribution
3.2.1. Carbohydrates
3.2.2. Proteins
3.2.3. Fats
3.2.4. Fibers
3.2.5. Sodium
3.2.6. Alcohol
3.2.7. Sweeteners
3.2.8. Micronutrients and Supplements
3.3. Nutritional Strategies
3.3.1. Mediterranean Diet
3.3.2. Dash (Dietary Approaches to Stop Hypertension)
3.3.3. Low-Carb Diet and Ketogenic Diet (Very Low-Carb Diet)
3.3.4. Low-Fat Diet
3.3.5. Ornish and Pritikin Diets (Very Low-Fat Diet)
3.3.6. Vegetarian and Vegan Diet (Plant-Based Diet)
3.3.7. Paleolithic Diet (Paleo Diet)
3.3.8. Intermittent Fasting
3.3.9. Mindful Eating Program
3.4. Summary of Dietary Patterns for the Management of T2DM
3.5. Nutritional Management of T2DM in the COVID-19 Pandemic
4. Authors’ Comments—The Best Nutritional Strategies and Dietary Prescription Recommended for the Management of T2DM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moulis, G.; Ibañez, B.; Palmaro, A.; Aizpuru, F.; Millan, E.; Lapeyre-Mestre, M.; Sailler, L.; Cambra, K. Cross-national health care database utilization between Spain and France: Results from the EPICHRONIC study assessing the prevalence of type 2 diabetes mellitus. Clin. Epidemiol. 2018, 10, 863–874. [Google Scholar] [CrossRef]
- Al-Jawaldeh, A.; Hammerich, A.; Doggui, R.; Engesveen, K.; Lang, K.; McColl, K. Implementation of WHO Recommended Policies and Interventions on Healthy Diet in the Countries of the Eastern Mediterranean Region: From Policy to Action. Nutrients 2020, 12, 3700. [Google Scholar] [CrossRef]
- Berger, S.E.; Huggins, G.S.; McCaffery, J.M.; Jacques, P.F.; Lichtenstein, A.H. Change in Cardiometabolic Risk Factors Associated with Magnitude of Weight Regain 3 Years after a 1-Year Intensive Lifestyle Intervention in Type 2 Diabetes Mellitus: The Look AHEAD Trial. J. Am. Heart Assoc. 2019, 8, e010951. [Google Scholar] [CrossRef]
- Magliano, D.J.; Boyko, E.J. IDF Diabetes Atlas 10th Edition Scientific Committee, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK581934/ (accessed on 20 August 2023).
- Belalcazar, L.M.; Ballantyne, C.M. Looking Back at Look AHEAD through the Lens of Recent Diabetes Outcome Trials. Circulation 2017, 135, 720–723. [Google Scholar] [CrossRef]
- Berger, S.E.; Huggins, G.S.; McCaffery, J.M.; Lichtenstein, A.H. Comparison among criteria to define successful weight-loss maintainers and regainers in the Action for Health in Diabetes (Look AHEAD) and Diabetes Prevention Program trials. Am. J. Clin. Nutr. 2017, 106, 1337–1346. [Google Scholar] [CrossRef]
- Look AHEAD Research Group. Impact of intensive lifestyle intervention on depression and health-related quality of life in type 2 diabetes: The Look AHEAD Trial. Diabetes Care 2014, 37, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154, Erratum in N. Engl. J. Med. 2014, 370, 1866. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40, Erratum in Diabetes Care 2023, 46, 1715. [Google Scholar] [CrossRef]
- Apolzan, J.W.; Venditti, E.M.; Edelstein, S.L.; Knowler, W.C.; Dabelea, D.; Boyko, E.J.; Pi-Sunyer, X.; Kalyani, R.R.; Franks, P.W.; Srikanthan, P.; et al. Long-Term Weight Loss with Metformin or Lifestyle Intervention in the Diabetes Prevention Program Outcomes Study. Ann. Intern. Med. 2019, 170, 682–690, Erratum in Ann. Intern. Med. 2020, 173, 508. [Google Scholar] [CrossRef]
- Gregg, E.W.; Jakicic, J.M.; Blackburn, G.; Bloomquist, P.; Bray, G.A.; Clark, J.M. Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J. Hypertens. 2018, 36, 1953–2041, Erratum in J. Hypertens. 2019, 37, 226. [Google Scholar] [CrossRef]
- Brazil, M.d.S. Food Guide for the Brazilian Population Promoting Healthy Eating. Standards and Technical Manuals. 2014, Brasília, 1–158. Available online: http://www.cfn.org.br/wp-content/uploads/2015/12/guia-alimentar-da-populacaobrasileira.pdf (accessed on 20 August 2023).
- Ivers, N.M.; Jiang, M.; Alloo, J.; Singer, A.; Ngui, D.; Casey, C.G.; Yu, C.H. Diabetes Canada 2018 clinical practice guidelines: Key messages for family physicians caring for patients living with type 2 diabetes. Can. Fam. Physician 2019, 65, 14–24. [Google Scholar]
- Connor, H.; Annan, F.; Bunn, E.; Frost, G.; McGough, N.; Sarwar, T.; Thomas, B. Nutrition Subcommittee of the Diabetes Care Advisory Committee of Diabetes, U.K. The implementation of nutritional advice for people with diabetes. Diabet. Med. 2003, 20, 786–807, Erratum in Diabet Med. 2004, 21, 200. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. ESC Scientific Document Group; 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140, Erratum in Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686, Erratum in Lancet 2009, 374, 2054. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Lindström, J.; Ilanne-Parikka, P.; Peltonen, M.; Aunola, S.; Eriksson, J.G.; Hemiö, K.; Hämäläinen, H.; Härkönen, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: Follow-up of the Finnish Diabetes Prevention Study. Lancet 2006, 368, 1673–1679. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Wang, J.; An, Y.; Gong, Q.; Gregg, E.W.; Yang, W.; Zhang, B.; Shuai, Y.; Hong, J.; et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: A 23-year follow-up study. Lancet Diabetes Endocrinol. 2014, 2, 474–480. [Google Scholar] [CrossRef]
- Evert, A.B.; Dennison, M.; Gardner, C.D.; Garvey, W.T.; Lau, K.H.K.; MacLeod, J.; Mitri, J.; Pereira, R.F.; Rawlings, K.; Robinson, S.; et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care 2019, 42, 731–754. [Google Scholar] [CrossRef]
- Carbone, S.; Dixon, D.L.; Buckley, L.F.; Abbate, A. Glucose-Lowering Therapies for Cardiovascular Risk Reduction in Type 2 Diabetes Mellitus: State-of-the-Art Review. Mayo Clin. Proc. 2018, 93, 1629–1647, Erratum in Mayo Clin. Proc. 2019, 94, 554. [Google Scholar] [CrossRef]
- Franz, M.J.; Bantle, J.P.; Beebe, C.A.; Brunzell, J.D.; Chiasson, J.L.; Garg, A.; Holzmeister, L.A.; Hoogwerf, B.; Mayer-Davis, E.; Mooradian, A.D.; et al. American Diabetes Association. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2003, 26 (Suppl. S1), S51–S61. [Google Scholar] [CrossRef]
- Sartorelli, D.S.; Sciarra, E.C.; Franco, L.J.; Cardoso, M.A. Primary prevention of type 2 diabetes through nutritional counseling. Diabetes Care 2004, 27, 3019. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children. 2012. Available online: http://www.ncbi.nlm.nih.gov/books/NBK133309/ (accessed on 20 September 2023).
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R.; et al. Management of hyperglycemia in type 2 diabetes, 2015: A patient-centered approach: Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef]
- Powers, M.A.; Bardsley, J.; Cypress, M.; Duker, P.; Funnell, M.M.; Fischl, A.H.; Maryniuk, M.D.; Siminerio, L.; Vivian, E. Diabetes Self-Management Education and Support in Type 2 Diabetes: A Joint Position Statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. J. Acad. Nutr. Diet. 2015, 115, 1323–1334. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Drehmer, M.; Odegaard, A.O.; Schmidt, M.I.; Duncan, B.B.; Cardoso, L.O.; Matos, S.M.A.; Molina, M.D.C.B.; Barreto, S.M.; Pereira, M.A. Brazilian dietary patterns and the dietary approaches to stop hypertension (DASH) diet-relationship with metabolic syndrome and newly diagnosed diabetes in the ELSA-Brasil study. Diabetol. Metab. Syndr. 2017, 9, 13. [Google Scholar] [CrossRef]
- Paula, T.P.; Viana, L.V.; Neto, A.T.; Leitão, C.B.; Gross, J.L.; Azevedo, M.J. Effects of the DASH Diet and Walking on Blood Pressure in Patients with Type 2 Diabetes and Uncontrolled Hypertension: A Randomized Controlled Trial. J. Clin. Hypertens. 2015, 17, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.J.; MacLeod, J.; Evert, A.; Brown, C.; Gradwell, E.; Handu, D.; Reppert, A.; Robinson, M. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J. Acad. Nutr. Diet. 2017, 117, 1659–1679. [Google Scholar] [CrossRef] [PubMed]
- Snetselaar, L.G.; de Jesus, J.M.; DeSilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef]
- Davidson, P.; Ross, T.; Castor, C. Academy of Nutrition and Dietetics: Revised 2017 Standards of Practice and Standards of Professional Performance for Registered Dietitian Nutritionists (Competent, Proficient, and Expert) in Diabetes Care. J. Acad. Nutr. Die. 2018, 118, 932–946.e48. [Google Scholar] [CrossRef]
- Briggs, E.K.; Stanley, K. Position of the Academy of Nutrition and Dietetics: The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes. J. Acad. Nutr. Diet 2018, 118, 343–353. [Google Scholar] [CrossRef] [PubMed]
- He, M.; van Dam, R.M.; Rimm, E.; Hu, F.B.; Qi, L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation 2010, 121, 2162–2168. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.N.; Beulens, J.W.; van der Schouw, Y.T.; Sluijs, I.; Spijkerman, A.M.; Sluik, D.; Boeing, H.; Kaaks, R.; Teucher, B.; Dethlefsen, C.; et al. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS ONE 2012, 7, e43127. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine; The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Am. Diet. Assoc. 2002, 102, 1621–1630, Erratum in J. Am. Diet Assoc. 2003, 103, 563. [Google Scholar] [CrossRef] [PubMed]
- Vega-López, S.; Venn, B.J.; Slavin, J.L. Relevance of the Glycemic Index and Glycemic Load for Body Weight, Diabetes, and Cardiovascular Disease. Nutrients 2018, 10, 1361. [Google Scholar] [CrossRef]
- Evert, A.B.; Boucher, J.L.; Cypress, M.; Dunbar, S.A.; Franz, M.J.; Mayer-Davis, E.J.; Neumiller, J.J.; Nwankwo, R.; Verdi, C.L.; Urbanski, P.; et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care 2014, 37 (Suppl. S1), S120–S143. [Google Scholar] [CrossRef]
- Oza-Frank, R.; Cheng, Y.J.; Narayan, K.M.; Gregg, E.W. Trends in nutrient intake among adults with diabetes in the United States: 1988–2004. J. Am. Diet Assoc. 2009, 109, 1173–1178. [Google Scholar] [CrossRef]
- Brand-Miller, J.C.; Stockmann, K.; Atkinson, F.; Petocz, P.; Denyer, G. Glycemic index, postprandial glycemia, and the shape of the curve in healthy subjects: Analysis of a database of more than 1,000 foods. Am. J. Clin. Nutr. 2009, 89, 97–105. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Dunbar, S.A.; Jaacks, L.M.; Karmally, W.; Mayer-Davis, E.J.; Wylie-Rosett, J.; Yancy, W.S., Jr. Macronutrients, food groups, and eating patterns in the management of diabetes: A systematic review of the literature, 2010. Diabetes Care 2012, 35, 434–445. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Shukla, A.P.; Dickison, M.; Coughlin, N.; Karan, A.; Mauer, E.; Truong, W.; Casper, A.; Emiliano, A.B.; Kumar, R.B.; Saunders, K.H.; et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes. Metab. 2019, 21, 377–381. [Google Scholar] [CrossRef]
- Brazilian Diabetes Society (SBD). Official Position on Therapeutic Conduct for People with Diabetes and Hypertension. Brazilian Diabetes Society (SBD). N03/2020. Arq. Bras Cardiol. 2020, 4, 1–20. Available online: https://profissional.diabetes.org.br/wp-content/uploads/2021/06/CONDUTA-TERAPEUTICA-HIPERTENSAO-ARTERIAL.pdf (accessed on 20 September 2023).
- Official Position of SBEM and ABESO on the Use of Coconut Oil to Lose Weight. Brazilian Society of Endocrinology and Metabology (SBEM); Brazilian Association for the Study of Obesity and Metabolic Syndrome (ABESO & SBEM). 2020. Available online: https://www.endocrino.org.br/media/uploads/posicionamento_oficial_%C3%B3leo_de_coco_sbem_e_abeso.pdf (accessed on 20 September 2023).
- Brazilian Diabetes Society. Carbohydrate Counting Manual; Department of Nutrition of the Brazilian Diabetes Society (SBD): Rio De Janeiro, Brazil, 2021; Available online: https://diabetes.org.br/wp-content/uploads/2021/05/manual-de-contagem-de-carbo.pdf (accessed on 20 September 2023).
- Gross, J.L.; Zelmanovitz, T.; Moulin, C.C.; De Mello, V.; Perassolo, M.; Leitão, C.; Hoefel, A.; Paggi, A.; Azevedo, M.J. Effect of a chicken-based diet on renal function and lipid profile in patients with type 2 diabetes: A randomized crossover trial. Diabetes Care 2002, 25, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Fuller, N.R.; Caterson, I.D.; Sainsbury, A.; Denyer, G.; Fong, M.; Gerofi, J.; Baqleh, K.; Williams, K.H.; Lau, N.S.; Markovic, T.P. The effect of a high-egg diet on cardiovascular risk factors in people with type 2 diabetes: The Diabetes and Egg (DIABEGG) study—A 3-mo randomized controlled trial. Am. J. Clin. Nutr. 2015, 101, 705–713. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary Protein Intake and Risk of Type 2 Diabetes in US Men and Women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Jenkins, A.L.; Brissette, C.; Choleva, L.; Jovanovski, E.; Gibbs, A.L.; Bazinet, R.P.; Au-Yeung, F.; Zurbau, A.; Ho, H.V.; et al. Salba-chia (Salvia hispanica L.) in the treatment of overweight and obese patients with type 2 diabetes: A double-blind randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 138–146. [Google Scholar] [CrossRef]
- Luger, M.; Holstein, B.; Schindler, K.; Kruschitz, R.; Ludvik, B. Feasibility and efficacy of an isocaloric high-protein vs. standard diet on insulin requirement, body weight and metabolic parameters in patients with type 2 diabetes on insulin therapy. Exp. Clin. Endocrinol. Diabetes 2013, 121, 286–294. [Google Scholar] [CrossRef]
- Dong, J.Y.; Zhang, Z.L.; Wang, P.Y.; Qin, L.Q. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: Meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 781–789. [Google Scholar] [CrossRef]
- Pfeiffer, A.F.H.; Pedersen, E.; Schwab, U.; Risérus, U.; Aas, A.M.; Uusitupa, M.; Thanopoulou, A.; Kendall, C.; Sievenpiper, J.L.; Kahleová, H.; et al. The Effects of Different Quantities and Qualities of Protein Intake in People with Diabetes Mellitus. Nutrients 2020, 12, 365. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Korat, A.A.; Malik, V.; Hu, F.B. Metabolic Effects of Monounsaturated Fatty Acid-Enriched Diets Compared with Carbohydrate or Polyunsaturated Fatty Acid-Enriched Diets in Patients with Type 2 Diabetes: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2016, 39, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Bendsen, N.T.; Christensen, R.; Bartels, E.M.; Astrup, A. Consumption of industrial and ruminant trans fatty acids and risk of coronary heart disease: A systematic review and meta-analysis of cohort studies. Eur. J. Clin. Nutr. 2011, 65, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209, Erratum in J. Am. Coll. Cardiol. 2019, 73, 3234–3237. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Jakicic, J.M.; Ard, J.D.; de Jesus, J.M.; Houston Miller, N.; Hubbard, V.S.; Lee, I.M.; Lichtenstein, A.H.; Loria, C.M.; Millen, B.E.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. S2), S76–S99, Erratum in Circulation 2015, 131, e326. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188, Erratum in Eur. Heart J. 2020, 41, 4255. [Google Scholar] [CrossRef]
- Izar, M.C.O.; Lottenberg, A.M.; Giraldez, V.Z.R.; Santos Filho, R.D.D.; Machado, R.M.; Bertolami, A.; Assad, M.H.V.; Saraiva, J.F.K.; Faludi, A.A.; Moreira, A.S.B.; et al. Position Statement on Fat Consumption and Cardiovascular Health—2021. Arq. Bras. Cardiol. 2021, 116, 160–212, Erratum in Arq. Bras. Cardiol. 2021, 116, 855. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Becerra-Tomás, N.; Ruiz-Canela, M.; Corella, D.; Schröder, H.; Estruch, R.; Ros, E.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Total and subtypes of dietary fat intake and risk of type 2 diabetes mellitus in the Prevención con Dieta Mediterránea (PREDIMED) study. Am. J. Clin. Nutr. 2017, 105, 723–735. [Google Scholar] [CrossRef]
- Salazar, J.; Angarita, L.; Morillo, V.; Navarro, C.; Martínez, M.S.; Chacín, M.; Torres, W.; Rajotia, A.; Rojas, M.; Cano, C.; et al. Microbiota and Diabetes Mellitus: Role of Lipid Mediators. Nutrients 2020, 12, 3039. [Google Scholar] [CrossRef]
- McNamara, D.J. Dietary cholesterol, heart disease risk and cognitive dissonance. Proc. Nutr. Soc. 2014, 73, 161–166. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. PREDIMED Study Investigators. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. American Heart Association. Dietary Fats and Cardiovascular Disease: A Presidential Advisory from the American Heart Association. Circulation 2017, 136, e1–e23, Erratum in Circulation 2017, 136, e195. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 8, CD011737. [Google Scholar] [CrossRef] [PubMed]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Prospective Urban Rural Epidemiology (PURE) study investigators. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef]
- McGuire, S. Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Departments of Agriculture and Health and Human Services, 2015. Adv. Nutr. 2016, 7, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Clinical Trials. Circulation 2020, 141, 803–814. [Google Scholar] [CrossRef]
- Sacks, F.M. Coconut Oil and Heart Health: Fact or Fiction? Circulation 2020, 141, 815–817. [Google Scholar] [CrossRef]
- Gijsbers, L.; Ding, E.L.; Malik, V.S.; de Goede, J.; Geleijnse, J.M.; Soedamah-Muthu, S.S. Consumption of dairy foods and diabetes incidence: A dose-response meta-analysis of observational studies. Am. J. Clin. Nutr. 2016, 103, 1111–1124. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Bogensberger, B.; Hoffmann, G. Diet Quality as Assessed by the Healthy Eating Index, Alternate Healthy Eating Index, Dietary Approaches to Stop Hypertension Score, and Health Outcomes: An Updated Systematic Review and Meta-Analysis of Cohort Studies. J. Acad. Nutr. Diet. 2018, 118, 74–100.e11. [Google Scholar] [CrossRef] [PubMed]
- Ericson, U.; Hellstrand, S.; Brunkwall, L.; Schulz, C.A.; Sonestedt, E.; Wallström, P.; Gullberg, B.; Wirfält, E.; Orho-Melander, M. Food sources of fat may clarify the inconsistent role of dietary fat intake for incidence of type 2 diabetes. Am. J. Clin. Nutr. 2015, 101, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Huo, R.; Du, T.; Xu, Y.; Xu, W.; Chen, X.; Sun, K.; Yu, X. Effects of Mediterranean-style diet on glycemic control, weight loss and cardiovascular risk factors among type 2 diabetes individuals: A meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with Mediterranean diets: A subgroup analysis of a randomized trial. Ann. Intern. Med. 2014, 160, 1–10, Erratum in Ann. Intern. Med. 2018, 169, 271–272. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Zare, M.; Faghih, S. Effects of Canola Oil Consumption on Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. J. Am. Coll. Nutr. 2019, 38, 185–196. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Matu, J.; Price, O.J.; Birch, K.M.; Ajjan, R.A.; Farrar, D.; Tapp, R.; West, D.J.; Deighton, K.; Campbell, M.D. Omega-3 polyunsaturated fatty acids favourably modulate cardiometabolic biomarkers in type 2 diabetes: A meta-analysis and meta-regression of randomized controlled trials. Cardiovasc. Diabetol. 2018, 17, 98. [Google Scholar] [CrossRef]
- Bosch, J.; Gerstein, H.C.; Dagenais, G.R.; Díaz, R.; Dyal, L.; Jung, H.; Maggiono, A.P.; Probstfield, J.; Ramachandran, A.; Riddle, M.C. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N. Engl. J. Med. 2012, 367, 309–318. [Google Scholar] [CrossRef]
- Wu, J.H.Y.; Marklund, M.; Imamura, F.; Tintle, N.; Ardisson Korat, A.V.; de Goede, J.; Zhou, X.; Yang, W.S.; de Oliveira Otto, M.C.; Kröger, J.; et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE). Omega-6 fatty acid biomarkers and incident type 2 diabetes: Pooled analysis of individual-level data for 39740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017, 5, 965–974. [Google Scholar] [CrossRef]
- Sawada, T.; Tsubata, H.; Hashimoto, N.; Takabe, M.; Miyata, T.; Aoki, K.; Yamashita, S.; Oishi, S.; Osue, T.; Yokoi, K.; et al. Effects of 6-month eicosapentaenoic acid treatment on postprandial hyperglycemia, hyperlipidemia, insulin secretion ability, and concomitant endothelial dysfunction among newly-diagnosed impaired glucose metabolism patients with coronary artery disease. An open label, single blinded, prospective randomized controlled trial. Cardiovasc. Diabetol. 2016, 15, 121. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- ASCEND Study Collaborative Group. Effects of n-3 Fatty Acid Supplements in Diabetes Mellitus. N. Engl. J. Med. 2018, 379, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. VITAL Research Group. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yu, X.; Shao, S. Effects of Omega-3 Fatty Acid Supplementation on Glucose Control and Lipid Levels in Type 2 Diabetes: A Meta-Analysis. PLoS ONE 2015, 10, e0139565. [Google Scholar] [CrossRef] [PubMed]
- Aronis, K.N.; Khan, S.M.; Mantzoros, C.S. Effects of trans fatty acids on glucose homeostasis: A meta-analysis of randomized, placebo-controlled clinical trials. Am. J. Clin. Nutr. 2012, 96, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Post, R.E.; Mainous, A.G., 3rd; King, D.E.; Simpson, K.N. Dietary fiber for the treatment of type 2 diabetes mellitus: A meta-analysis. J. Am. Board Fam. Med. 2012, 25, 16–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Cogswell, M.E.; Gillespie, C.; Fang, J.; Loustalot, F.; Dai, S.; Carriquiry, A.L.; Kuklina, E.V.; Hong, Y.; Merritt, R.; et al. Association between usual sodium and potassium intake and blood pressure and hypertension among U.S. adults: NHANES 2005–2010. PLoS ONE 2013, 8, e75289. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). CDC grand rounds: Dietary sodium reduction—Time for choice. Morb. Mortal. Wkly. Rep. 2012, 61, 89–91. [Google Scholar]
- Appel, L.J.; Frohlich, E.D.; Hall, J.E.; Pearson, T.A.; Sacco, R.L.; Seals, D.R.; Sacks, F.M.; Smith, S.C., Jr.; Vafiadis, D.K.; Van Horn, L.V. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: A call to action from the American Heart Association. Circulation 2011, 123, 1138–1143. [Google Scholar] [CrossRef]
- Azadbakht, L.; Fard, N.R.; Karimi, M.; Baghaei, M.H.; Surkan, P.J.; Rahimi, M.; Esmaillzadeh, A.; Willett, W.C. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: A randomized crossover clinical trial. Diabetes Care 2011, 34, 55–57. [Google Scholar] [CrossRef]
- Ekinci, E.I.; Clarke, S.; Thomas, M.C.; Moran, J.L.; Cheong, K.; MacIsaac, R.J.; Jerums, G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care 2011, 34, 703–709. [Google Scholar] [CrossRef]
- Thomas, M.C.; Moran, J.; Forsblom, C.; Harjutsalo, V.; Thorn, L.; Ahola, A.; Wadén, J.; Tolonen, N.; Saraheimo, M.; Gordin, D.; et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care 2011, 34, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Dunkler, D.; Dehghan, M.; Teo, K.K.; Heinze, G.; Gao, P.; Kohl, M.; Clase, C.M.; Mann, J.F.; Yusuf, S.; Oberbauer, R. ONTARGET Investigators. Diet and kidney disease in high-risk individuals with type 2 diabetes mellitus. JAMA Intern. Med. 2013, 173, 1682–1692. [Google Scholar] [CrossRef] [PubMed]
- Maillot, M.; Drewnowski, A. A conflict between nutritionally adequate diets and meeting the 2010 dietary guidelines for sodium. Am. J. Prev. Med. 2012, 42, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Wainstein, J.; Harman-Boehm, I.; Raz, I.; Fraser, D.; Rudich, A.; Stampfer, M.J. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: A multicenter, randomized, clinical intervention trial. Diabetes Care 2007, 30, 3011–3016. [Google Scholar] [CrossRef]
- Ahmed, A.T.; Karter, A.J.; Warton, E.M.; Doan, J.U.; Weisner, C.M. The relationship between alcohol consumption and glycemic control among patients with diabetes: The Kaiser Permanente Northern California Diabetes Registry. J. Gen. Intern. Med. 2008, 23, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bantle, A.E.; Thomas, W.; Bantle, J.P. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism 2008, 57, 241–245. [Google Scholar] [CrossRef]
- Schrieks, I.C.; Heil, A.L.; Hendriks, H.F.; Mukamal, K.J.; Beulens, J.W. The effect of alcohol consumption on insulin sensitivity and glycemic status: A systematic review and meta-analysis of intervention studies. Diabetes Care 2015, 38, 723–732. [Google Scholar] [CrossRef]
- Howard, A.A.; Arnsten, J.H.; Gourevitch, M.N. Effect of alcohol consumption on diabetes mellitus: A systematic review. Ann. Intern. Med. 2004, 140, 211–219. [Google Scholar] [CrossRef]
- Timko, C.; Kong, C.; Vittorio, L.; Cucciare, M.A. Screening and brief intervention for unhealthy substance use in patients with chronic medical conditions: A systematic review. J. Clin. Nurs. 2016, 25, 3131–3143. [Google Scholar] [CrossRef]
- Gepner, Y.; Golan, R.; Harman-Boehm, I.; Henkin, Y.; Schwarzfuchs, D.; Shelef, I.; Durst, R.; Kovsan, J.; Bolotin, A.; Leitersdorf, E.; et al. Effects of Initiating Moderate Alcohol Intake on Cardiometabolic Risk in Adults with Type 2 Diabetes: A 2-Year Randomized, Controlled Trial. Ann. Intern. Med. 2015, 163, 569–579. [Google Scholar] [CrossRef]
- Gepner, Y.; Henkin, Y.; Schwarzfuchs, D.; Golan, R.; Durst, R.; Shelef, I.; Harman-Boehm, I.; Spitzen, S.; Witkow, S.; Novack, L.; et al. Differential Effect of Initiating Moderate Red Wine Consumption on 24-h Blood Pressure by Alcohol Dehydrogenase Genotypes: Randomized Trial in Type 2 Diabetes. Am. J. Hypertens. 2016, 29, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, T.; Wakabayashi, I. Inverse associations between light-to-moderate alcohol intake and lipid-related indices in patients with diabetes. Cardiovasc. Diabetol. 2013, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Tetzschner, R.; Nørgaard, K.; Ranjan, A. Effects of alcohol on plasma glucose and prevention of alcohol-induced hypoglycemia in type 1 diabetes-A systematic review with GRADE. Diabetes Metab. Res. Rev. 2018, 34, e2965. [Google Scholar] [CrossRef] [PubMed]
- Barnard, K.D.; Dyson, P.; Sinclair, J.M.; Lawton, J.; Anthony, D.; Cranston, M.; Holt, R.I. Alcohol health literacy in young adults with type 1 diabetes and its impact on diabetes management. Diabet. Med. 2014, 31, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, D.O.; Taylor, B.J.; Irving, H.; Roerecke, M.; Patra, J.; Mohapatra, S.; Rehm, J. Alcohol as a risk factor for type 2 diabetes: A systematic review and meta-analysis. Diabetes Care 2009, 32, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Knott, C.; Bell, S.; Britton, A. Alcohol Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of More Than 1.9 Million Individuals from 38 Observational Studies. Diabetes Care 2015, 38, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Zhang, Y. Specific types of alcoholic beverage consumption and risk of type 2 diabetes: A systematic review and meta-analysis. J. Diabetes Investig. 2017, 8, 56–68. [Google Scholar] [CrossRef]
- Pan, A.; Malik, V.S.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am. J. Clin. Nutr. 2012, 95, 1454–1460. [Google Scholar] [CrossRef]
- Malik, V.S. Sugar sweetened beverages and cardiometabolic health. Curr. Opin. Cardiol. 2017, 32, 572–579. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health: What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef]
- Imamura, F.; O’Connor, L.; Ye, Z.; Mursu, J.; Hayashino, Y.; Bhupathiraju, S.N.; Forouhi, N.G. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: Systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 2015, 351, h3576. [Google Scholar] [CrossRef]
- Richardson, I.L.; Frese, S.A. Non-nutritive sweeteners and their impacts on the gut microbiome and host physiology. Front. Nutr. 2022, 9, 988144. [Google Scholar] [CrossRef]
- Brazil. Resolution No. 818, 28 September 2023. Provides for the Health Requirements for Tabletop Sweeteners and Dietary Sweeteners. Published in the DOU—Official Gazette of the Union; Executive Branch, No. 188, of October 2, 2023. Available online: https://antigo.anvisa.gov.br/documents/10181/6661634/RDC_818_2023_.pdf/0b734a91-e7ff-43d6-b29f-98528d2b0a7d#:~:text=Disp%C3%B5e%20sobre%20os%20requisitos%20sanit%C3%A1rios,que%20lhe%20conferem%20os%20arts. (accessed on 29 October 2023).
- Johnson, R.K.; Appel, L.J.; Brands, M.; Howard, B.V.; Lefevre, M.; Lustig, R.H. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young; American Diabetes Association. Nonnutritive sweeteners: Current use and health perspectives: A scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2012, 35, 1798–1808. [Google Scholar] [CrossRef]
- Johnson, R.K.; Lichtenstein, A.H.; Anderson, C.A.M.; Carson, J.A.; Després, J.P.; Hu, F.B.; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Quality of Care and Outcomes Research; et al. Low-Calorie Sweetened Beverages and Cardiometabolic Health: A Science Advisory from the American Heart Association. Circulation 2018, 138, e126–e140. [Google Scholar] [CrossRef] [PubMed]
- Nichol, A.D.; Holle, M.J.; An, R. Glycemic impact of non-nutritive sweeteners: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2018, 72, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Sylvetsky, A.C.; Rother, K.I. Nonnutritive Sweeteners in Weight Management and Chronic Disease: A Review. Obesity 2018, 26, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, N.; Padwal, R.; Field, C.; Marks, S.; Jacobs, R.; Tonelli, M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E.; Gaziano, J.M. Multivitamins in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2012, 308, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, H.; Pipingas, A.; Pase, M.P. Multivitamin-multimineral supplementation and mortality: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2013, 97, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef]
- Buvat, D.R. Use of metformin is a cause of vitamin B12 deficiency. Am. Fam. Physician 2004, 69, 264. [Google Scholar] [PubMed]
- Bauman, W.A.; Shaw, S.; Jayatilleke, E.; Spungen, A.M.; Herbert, V. Increased intake of calcium reverses vitamin B12 malabsorption induced by metformin. Diabetes Care 2000, 23, 1227–1231. [Google Scholar] [CrossRef]
- Wang, H.; Li, L.; Qin, L.L.; Song, Y.; Vidal-Alaball, J.; Liu, T.H. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst. Rev. 2018, 3, CD004655. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Vidal-Alaball, J.; Cannings-John, R.; McCaddon, A.; Hood, K.; Papaioannou, A.; McDowell, I.; Goringe, A. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: A systematic review of randomized controlled trials. Fam. Pract. 2006, 23, 279–285. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes-2023. Diabetes Care 2023, 46 (Suppl. S1), S68–S96. [Google Scholar] [CrossRef] [PubMed]
- Balk, E.M.; Tatsioni, A.; Lichtenstein, A.H.; Lau, J.; Pittas, A.G. Effect of chromium supplementation on glucose metabolism and lipids: A systematic review of randomized controlled trials. Diabetes Care 2007, 30, 2154–2163. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cotillard, A.; Vatier, C.; Bastard, J.P.; Fellahi, S.; Stévant, M.; Allatif, O.; Langlois, C.; Bieuvelet, S.; Brochot, A.; et al. A Dietary Supplement Containing Cinnamon, Chromium and Carnosine Decreases Fasting Plasma Glucose and Increases Lean Mass in Overweight or Obese Pre-Diabetic Subjects: A Randomized, Placebo-Controlled Trial. PLoS ONE 2015, 10, e0138646, Erratum in PLoS ONE 2015, 10, e0145315. [Google Scholar] [CrossRef]
- Veronese, N.; Watutantrige-Fernando, S.; Luchini, C.; Solmi, M.; Sartore, G.; Sergi, G.; Manzato, E.; Barbagallo, M.; Maggi, S.; Stubbs, B. Effect of magnesium supplementation on glucose metabolism in people with or at risk of diabetes: A systematic review and meta-analysis of double-blind randomized controlled trials. Eur. J. Clin. Nutr. 2016, 70, 1354–1359, Erratum in Eur. J. Clin. Nutr. 2016, 70, 1463. [Google Scholar] [CrossRef] [PubMed]
- De Valk, H.W.; Verkaaik, R.; van Rijn, H.J.; Geerdink, R.A.; Struyvenberg, A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet. Med. 1998, 15, 503–507. [Google Scholar] [CrossRef]
- Jorde, R.; Figenschau, Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25-hydroxyvitamin D levels. Eur. J. Nutr. 2009, 48, 349–354. [Google Scholar] [CrossRef]
- Patel, P.; Poretsky, L.; Liao, E. Lack of effect of subtherapeutic vitamin D treatment on glycemic and lipid parameters in Type 2 diabetes: A pilot prospective randomized trial. J. Diabetes. 2010, 2, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.; Sarathi, V.; Shivane, V.K.; Bandgar, T.R.; Menon, P.S.; Shah, N.S. Pilot study to evaluate the effect of short-term improvement in vitamin D status on glucose tolerance in patients with type 2 diabetes mellitus. Endocr. Pract. 2010, 16, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Neyestani, T.R.; Farvid, M.; Alavi-Majd, H.; Houshiarrad, A.; Kalayi, A.; Shariatzadeh, N.; Gharavi, A.; Heravifard, S.; Tayebinejad, N.; et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: A randomized clinical trial. Am. J. Clin. Nutr. 2011, 93, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Soric, M.M.; Renner, E.T.; Smith, S.R. Effect of daily vitamin D supplementation on HbA1c in patients with uncontrolled type 2 diabetes mellitus: A pilot study. J. Diabetes 2012, 4, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Al-Daghri, N.M.; Sabico, S.B.; Al-Othman, A.; Moharram, O.; Alokail, M.S.; Al-Saleh, Y.; Kumar, S.; Chrousos, G.P. Vitamin D supplementation in patients with diabetes mellitus type 2 on different therapeutic regimens: A one-year prospective study. Cardiovasc. Diabetol. 2013, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Sadiya, A.; Ahmed, S.M.; Carlsson, M.; Tesfa, Y.; George, M.; Ali, S.H.; Siddieg, H.H.; Abusnana, S. Vitamin D3 supplementation and body composition in persons with obesity and type 2 diabetes in the UAE: A randomized controlled double-blinded clinical trial. Clin. Nutr. 2016, 35, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.; Naderpoor, N.; de Courten, M.P.; Teede, H.; Kellow, N.; Walker, K.; Scragg, R.; de Courten, B. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D-deficient, overweight or obese adults: A randomized placebo-controlled trial. Am. J. Clin. Nutr. 2017, 105, 1372–1381. [Google Scholar] [CrossRef]
- Moreira-Lucas, T.S.; Duncan, A.M.; Rabasa-Lhoret, R.; Vieth, R.; Gibbs, A.L.; Badawi, A.; Wolever, T.M. Effect of vitamin D supplementation on oral glucose tolerance in individuals with low vitamin D status and increased risk for developing type 2 diabetes (EVIDENCE): A double-blind, randomized, placebo-controlled clinical trial. Diabetes Obes. Metab. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Tabesh, M.; Azadbakht, L.; Faghihimani, E.; Tabesh, M.; Esmaillzadeh, A. Effects of calcium-vitamin D co-supplementation on metabolic profiles in vitamin D insufficient people with type 2 diabetes: A randomised controlled clinical trial. Diabetologia 2014, 57, 2038–2047. [Google Scholar] [CrossRef]
- Solis, M.Y.; Artioli, G.G.; Gualano, B. Potential of Creatine in Glucose Management and Diabetes. Nutrients 2021, 13, 570. [Google Scholar] [CrossRef]
- Chedid, V.; Dhalla, S.; Clarke, J.O.; Roland, B.C.; Dunbar, K.B.; Koh, J.; Justino, E.; Tomakin, E.; Mullin, G.E. Herbal therapy is equivalent to rifaximin for the treatment of small intestinal bacterial overgrowth. Glob. Adv. Health Med. 2014, 3, 16–24. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Dietary Supplements. 2018. Available online: https://www.fda.gov/food/dietarysupplements/ (accessed on 1 October 2023).
- Hidalgo-Mora, J.J.; García-Vigara, A.; Sánchez-Sánchez, M.L.; García-Pérez, M.Á.; Tarín, J.; Cano, A. The Mediterranean diet: A historical perspective on food for health. Maturitas 2020, 132, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Lindström, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Stentz, F.B.; Brewer, A.; Wan, J.; Garber, C.; Daniels, B.; Sands, C.; Kitabchi, A.E. Remission of pre-diabetes to normal glucose tolerance in obese adults with high protein versus high carbohydrate diet: Randomized control trial. BMJ Open Diabetes Res. Care 2016, 4, e000258. [Google Scholar] [CrossRef] [PubMed]
- Esposito, K.; Chiodini, P.; Maiorino, M.I.; Bellastella, G.; Panagiotakos, D.; Giugliano, D. Which diet for prevention of type 2 diabetes? A meta-analysis of prospective studies. Endocrine 2014, 47, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.H.T.; Pan, W.H.; Lin, M.N.; Lin, C.L. Vegetarian diet, change in dietary patterns, and diabetes risk: A prospective study. Nutr. Diabetes. 2018, 8, 12. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Díaz-López, A.; Rosique-Esteban, N.; Ros, E.; Buil-Cosiales, P.; Corella, D.; Estruch, R.; Fitó, M.; Serra-Majem, L.; Arós, F.; et al. PREDIMED Study Investigators. Legume consumption is inversely associated with type 2 diabetes incidence in adults: A prospective assessment from the PREDIMED study. Clin. Nutr. 2018, 37, 906–913. [Google Scholar] [CrossRef]
- Lee, Y.; Park, K. Adherence to a Vegetarian Diet and Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 603. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Hoffmann, G.; Schwedhelm, C.; Boeing, H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur. J. Epidemiol. 2018, 33, 157–170. [Google Scholar] [CrossRef]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030, Erratum in PLoS ONE 2019, 14, e0212203. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef]
- Anderssen, S.A.; Hjermann, I.; Urdal, P.; Torjesen, P.A.; Holme, I. Improved carbohydrate metabolism after physical training and dietary intervention in individuals with the “atherothrombogenic syndrome’. Oslo Diet and Exercise Study (ODES). A randomized trial. J. Intern. Med. 1996, 240, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villar, C.; Pérez-Heras, A.; Mercadé, I.; Casals, E.; Ros, E. Comparison of a high-carbohydrate and a high-monounsaturated fat, olive oil-rich diet on the susceptibility of LDL to oxidative modification in subjects with Type 2 diabetes mellitus. Diabet. Med. 2004, 21, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Brazionis, L.; Kaimakamis, M.; Cameron, M.; Best, J.D.; O’Dea, K.; Rowley, K. Can the Mediterranean diet lower HbA1c in type 2 diabetes? Results from a randomized cross-over study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Toobert, D.J.; Glasgow, R.E.; Strycker, L.A.; Barrera, M., Jr.; Radcliffe, J.L.; Wander, R.C.; Bagdade, J.D. Biologic and quality-of-life outcomes from the Mediterranean Lifestyle Program: A randomized clinical trial. Diabetes Care 2003, 26, 2288–2293. [Google Scholar] [CrossRef]
- Elhayany, A.; Lustman, A.; Abel, R.; Attal-Singer, J.; Vinker, S. A low carbohydrate Mediterranean diet improves cardiovascular risk factors and diabetes control among overweight patients with type 2 diabetes mellitus: A 1-year prospective randomized intervention study. Diabetes Obes. Metab. 2010, 12, 204–209. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ciotola, M.; Di Palo, C.; Scognamiglio, P.; Gicchino, M.; Petrizzo, M.; Saccomanno, F.; Beneduce, F.; Ceriello, A.; et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: A randomized trial. Ann. Intern. Med. 2009, 151, 306–314, Erratum in Ann. Intern. Med. 2009, 151, 591. [Google Scholar] [CrossRef]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652, Erratum in Am. J. Clin. Nutr. 2022, 116, 1904. [Google Scholar] [CrossRef]
- Tay, J.; Thompson, C.H.; Luscombe-Marsh, N.D.; Wycherley, T.P.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Effects of an energy-restricted low-carbohydrate, high unsaturated fat/low saturated fat diet versus a high-carbohydrate, low-fat diet in type 2 diabetes: A 2-year randomized clinical trial. Diabetes Obes. Metab. 2018, 20, 858–871. [Google Scholar] [CrossRef]
- Sainsbury, E.; Kizirian, N.V.; Partridge, S.R.; Gill, T.; Colagiuri, S.; Gibson, A.A. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: A systematic review and meta-analysis. Diabetes Res. Clin. Pract. 2018, 139, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of low-carbohydrate- compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including GRADE assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef] [PubMed]
- Bhanpuri, N.H.; Hallberg, S.J.; Williams, P.T.; McKenzie, A.L.; Ballard, K.D.; Campbell, W.W.; McCarter, J.P.; Phinney, S.D.; Volek, J.S. Cardiovascular disease risk factor responses to a type 2 diabetes care model including nutritional ketosis induced by sustained carbohydrate restriction at 1 year: An open label, non-randomized, controlled study. Cardiovasc. Diabetol. 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.; Luscombe-Marsh, N.D.; Thompson, C.H.; Noakes, M.; Buckley, J.D.; Wittert, G.A.; Yancy, W.S., Jr.; Brinkworth, G.D. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: A randomized trial. Am. J. Clin. Nutr. 2015, 102, 780–790. [Google Scholar] [CrossRef]
- Wycherley, T.P.; Thompson, C.H.; Buckley, J.D.; Luscombe-Marsh, N.D.; Noakes, M.; Wittert, G.A.; Brinkworth, G.D. Long-term effects of weight loss with a very-low carbohydrate, low saturated fat diet on flow mediated dilatation in patients with type 2 diabetes: A randomised controlled trial. Atherosclerosis 2016, 252, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.B.; Brancati, F.L.; Chen, H.; Coday, M.; Jakicic, J.M.; Lewis, C.E.; Stewart, K.J.; Clark, J.M.; Look AHEAD Research Group. Effect of improved fitness beyond weight loss on cardiovascular risk factors in individuals with type 2 diabetes in the Look AHEAD study. Eur. J Prev. Cardiol. 2014, 21, 608–617. [Google Scholar] [CrossRef]
- Pi-Sunyer, X.; Blackburn, G.; Brancati, F.L.; Bray, G.A.; Bright, R.; Clark, J.M.; Curtis, J.M.; Espeland, M.A.; Foreyt, J.P.; Graves, K.; et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: One-year results of the look AHEAD trial. Diabetes Care 2007, 30, 1374–1383. [Google Scholar] [CrossRef]
- Brehm, B.J.; Lattin, B.L.; Summer, S.S.; Boback, J.A.; Gilchrist, G.M.; Jandacek, R.J.; D’Alessio, D.A. One-year comparison of a high-monounsaturated fat diet with a high-carbohydrate diet in type 2 diabetes. Diabetes Care 2009, 32, 215–220. [Google Scholar] [CrossRef]
- Davis, N.J.; Tomuta, N.; Schechter, C.; Isasi, C.R.; Segal-Isaacson, C.J.; Stein, D.; Zonszein, J.; Wylie-Rosett, J. Comparative study of the effects of a 1-year dietary intervention of a low-carbohydrate diet versus a low-fat diet on weight and glycemic control in type 2 diabetes. Diabetes Care 2009, 32, 1147–1152. [Google Scholar] [CrossRef]
- Guldbrand, H.; Dizdar, B.; Bunjaku, B.; Lindström, T.; Bachrach-Lindström, M.; Fredrikson, M.; Ostgren, C.J.; Nystrom, F.H. In type 2 diabetes, randomisation to advice to follow a low-carbohydrate diet transiently improves glycaemic control compared with advice to follow a low-fat diet producing a similar weight loss. Diabetologia 2012, 55, 2118–2127. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Triantafillidou, D.; Panagiotakos, D.B.; Koutsovasilis, A.; Saliaris, M.; Manolis, A.; Melidonis, A.; Zampelas, A. A high-protein low-fat diet is more effective in improving blood pressure and triglycerides in calorie-restricted obese individuals with newly diagnosed type 2 diabetes. Eur. J. Clin. Nutr. 2010, 64, 595–602. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Sato, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.J.; Massey, M.R.; Cherny, S.; O’Brien, L.T.; Pritikin, N. Long-term use of a high-complex-carbohydrate, high-fiber, low-fat diet and exercise in the treatment of NIDDM patients. Diabetes Care 1983, 6, 268–273. [Google Scholar] [CrossRef]
- Barnard, R.J.; Jung, T.; Inkeles, S.B. Diet and exercise in the treatment of NIDDM. The need for early emphasis. Diabetes Care. 1994, 17, 1469–1472. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.S.; Sklar, M.; Barnard, N.D.; Gore, S.; Sullivan, R.; Browning, S. Toward improved management of NIDDM: A randomized, controlled, pilot intervention using a lowfat, vegetarian diet. Prev. Med. 1999, 29, 87–91. [Google Scholar] [CrossRef]
- Tonstad, S.; Butler, T.; Yan, R.; Fraser, G.E. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care 2009, 32, 791–796. [Google Scholar] [CrossRef]
- Kahleova, H.; Matoulek, M.; Malinska, H.; Oliyarnik, O.; Kazdova, L.; Neskudla, T.; Skoch, A.; Hajek, M.; Hill, M.; Kahle, M.; et al. Vegetarian diet improves insulin resistance and oxidative stress markers more than conventional diet in subjects with Type 2 diabetes. Diabet. Med. 2011, 28, 549–559. [Google Scholar] [CrossRef]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Hosseinpour-Niazi, S.; Mirmiran, P.; Hedayati, M.; Azizi, F. Substitution of red meat with legumes in the therapeutic lifestyle change diet based on dietary advice improves cardiometabolic risk factors in overweight type 2 diabetes patients: A cross-over randomized clinical trial. Eur. J. Clin. Nutr. 2015, 69, 592–597. [Google Scholar] [CrossRef]
- McMacken, M.; Shah, S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017, 14, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Pollakova, D.; Andreadi, A.; Pacifici, F.; Della-Morte, D.; Lauro, D.; Tubili, C. The Impact of Vegan Diet in the Prevention and Treatment of Type 2 Diabetes: A Systematic Review. Nutrients 2021, 13, 2123. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, T.; Granfeldt, Y.; Ahrén, B.; Branell, U.C.; Pålsson, G.; Hansson, A.; Söderström, M.; Lindeberg, S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: A randomized cross-over pilot study. Cardiovasc. Diabetol. 2009, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Masharani, U.; Sherchan, P.; Schloetter, M.; Stratford, S.; Xiao, A.; Sebastian, A.; Nolte Kennedy, M.; Frassetto, L. Metabolic and physiologic effects from consuming a hunter-gatherer (Paleolithic)-type diet in type 2 diabetes. Eur. J. Clin. Nutr. 2015, 69, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Lindeberg, S.; Jönsson, T.; Granfeldt, Y.; Borgstrand, E.; Soffman, J.; Sjöström, K.; Ahrén, B. A Palaeolithic diet improves glucose tolerance more than a Mediterranean-like diet in individuals with ischaemic heart disease. Diabetologia 2007, 50, 1795–1807. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Clifton, P.M.; Keogh, J.B. Effect of Intermittent Compared with Continuous Energy Restricted Diet on Glycemic Control in Patients with Type 2 Diabetes: A Randomized Noninferiority Trial. JAMA Netw. Open. 2018, 1, e180756. [Google Scholar] [CrossRef]
- Corley, B.T.; Carroll, R.W.; Hall, R.M.; Weatherall, M.; Parry-Strong, A.; Krebs, J.D. Intermittent fasting in Type 2 diabetes mellitus and the risk of hypoglycaemia: A randomized controlled trial. Diabet. Med. 2018, 35, 588–594. [Google Scholar] [CrossRef]
- Borgundvaag, E.; Mak, J.; Kramer, C.K. Metabolic Impact of Intermittent Fasting in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis of Interventional Studies. J. Clin. Endocrinol. Metab. 2021, 106, 902–911. [Google Scholar] [CrossRef]
- Miller, C.K. Mindful Eating with Diabetes. Diabetes Spectr. 2017, 30, 89–94. [Google Scholar] [CrossRef]
- Mason, A.E.; Saslow, L.; Moran, P.J.; Kim, S.; Wali, P.K.; Abousleiman, H.; Hartman, A.; Richler, R.; Schleicher, S.; Hartogensis, W.; et al. Examining the Effects of Mindful Eating Training on Adherence to a Carbohydrate-Restricted Diet in Patients with Type 2 Diabetes (the DELISH Study): Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2019, 8, e11002, Erratum in JMIR Res. Protoc. 2020, 9, e17226. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Roso, M.B.; Knott-Torcal, C.; Matilla-Escalante, D.C.; Garcimartín, A.; Sampedro-Nuñez, M.A.; Dávalos, A.; Marazuela, M. COVID-19 Lockdown and Changes of the Dietary Pattern and Physical Activity Habits in a Cohort of Patients with Type 2 Diabetes Mellitus. Nutrients 2020, 12, 2327. [Google Scholar] [CrossRef] [PubMed]
- Grabia, M.; Markiewicz-Żukowska, R.; Puścion-Jakubik, A.; Bielecka, J.; Nowakowski, P.; Gromkowska-Kępka, K.; Mielcarek, K.; Socha, K. The Nutritional and Health Effects of the COVID-19 Pandemic on Patients with Diabetes Mellitus. Nutrients 2020, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Sankar, P.; Ahmed, W.N.; Mariam Koshy, V.; Jacob, R.; Sasidharan, S. Effects of COVID-19 lockdown on type 2 diabetes, lifestyle and psychosocial health: A hospital-based cross-sectional survey from South India. Diabetes Metab. Syndr. 2020, 14, 1815–1819. [Google Scholar] [CrossRef] [PubMed]
| Dietary Patterns | Description | Level of Evidence | Adherence and Comments |
|---|---|---|---|
| Mediterranean Diet | Mediterranean diet encourages consumption of foods with high nutritional quality, such as fruits, vegetables, legumes, whole grains, cereals, tubers, roots fish, low-fat dairy products, vegetable oils, nuts, seed oils, and wine in moderation [16,132,163,164,165,166,167]. | High | (1) High long-term adherence [16,132,163,164,165,166,167]. (2) There is a lot of evidence in the literature for the improvement of molecular markers, glycemic control, cardiovascular health, and weight loss [16,132,163,164,165,166,167]. |
| Dash Diet (Dietary Approaches to Stop Hypertension) | DASH diet is similar to the Mediterranean pattern, but alcohol consumption is not encouraged and there is also sodium restriction. It consists of fruits, vegetables, legumes, whole grains, cereals, tubers, roots, fish, chicken breast, lean meats, low-fat dairy products, vegetable oils, nuts, oilseeds and sodium restriction (<2300 mg or 1500 mg for severe heart disease) [16,30,31,63,95,132]. | High | (1) High long-term adherence [16,30,31,63,95,132]. (2) There is a lot of evidence in the literature for the improvement of molecular markers, glycemic control, cardiovascular health, reduce blood pressure and weight loss [16,30,31,63,95,132]. (3) Severe sodium restriction (1500 mg) requires caution and monitoring, and is indicated for some specific cases [16,30,31,63,94,95,96,97,98,99,132]. |
| Ketogenic Diet (very low-carb diet) | A dietary pattern composed of 5–10% carbohydrates, 15–25% proteins and 60–70% fats of the total energy value (TEV). Includes raw vegetables, very low-carb fruits (avocado and strawberries), all types of meat (beef, pork, fish and chicken), full-fat dairy products, vegetable oils, animal fat and eggs. It has a low intake of fruits, legumes, whole grains, cereals, roots and tubers [16,132,167,173]. | Low/ Moderate | (1) Low long-term adherence [16,132,167,168,169,170,171,172,173,174]. (2) There are many controversies in the literature [16,132,167,168,169,170,171,172,173,174]. (3) No advantages over other nutritional strategies [16,132,167]. (4) The severe carbohydrate restriction may not really be necessary and safe [16,132,167]. (5) New evidence shows results that it improves weight loss and glycemic control in the short term, but increases markers of cardiovascular risk, such as LDL cholesterol [16,132,167]. (6) This dietary pattern also can increase the risk of dehydration and hypoglycemia; there is a high probability of food monotony, low fiber and micronutrients intake [16,132,167]. (7) More studies are needed in patients with diabetes, especially in the long term [16,132,167]. |
| Low-Carb Diet | Low-carb diet promotes reducing the consumption of ultra-processed foods. The carbohydrate intake range is 40–45% of the TEV. Encourages the consumption of fruits, vegetables, legumes, whole grains, tubers, fish, lean meats, skimmed dairy products, vegetable oils, nuts, avocados, eggs and seed oils. Carbohydrates of high nutritional quality are allowed, but without excess [16,132,167,168,169,170,171,172,173,174]. | High | (1) High long-term adherence [16,132,167]. (2) In recent years, good evidence has been published in the literature for the modulation of molecular markers, glycemic control, cardiovascular health and weight loss [16,132,167,168,169,170,171,172,173,174]. (3) There are several types of protocols, and those that restrict saturated fats (<7% of TEV) and prioritize sources of polyunsaturated and monounsaturated fats show good results [16,132,167,168,169,170,171,172,173,174]. |
| Low-Fat | This dietary pattern involves ingesting 25–30% fats within the TEV. It encourages the consumption of fruits, vegetables, legumes, whole grains, tubers, fish, chicken breast, lean meats, and skimmed dairy products. This eating pattern is similar to DASH [175,176,177,178,179,180,181]. | High | (1) High long-term adherence [175,176,177,178,179,180,181]. (2) There is a lot of evidence in the literature for the modulation of molecular markers, glycemic control, cardiovascular health, reduced blood pressure, and weight loss [175,176,177,178,179,180,181]. |
| Ornish and Pritikins (very low-fat diet) | Both eating patterns have very low fat consumption (10% fat of the TEV). They encourage the consumption of whole foods, vegetables, legumes, fruits, grains, low-fat dairy products, and egg whites [16,132,182,183,184,185]. | Low | (1) Low long-term adherence [16,132,182,183,184,185]. (2) Over the years, the scientific community has lost interest in studying this type of diet, since it has low adherence, small palatability, and can possibly cause metabolic damage (hormone production, protection, and energy storage) [16,132,182,183,184,185]. |
| Plant-Based Diet (or vegetarian/ vegan diet) | Plant-based diet consisting eat of foods with high nutritional quality, such as fruits, vegetables, legumes, whole grains, cereal, tubers, roots, vegetable oils, nuts and seed oils. In this diet there is no intake of any type of food from an animal source (intake of high-fiber foods). It encourages questioning about food choices, autonomy, and ethical and cultural issues [16,132,184,185,186,187,188,189,190,191]. | High | (1) Moderate long-term adherence [16,132,184,185,186,187,188,189,190,191]. (2) There is a lot of evidence in the literature for the modulation of molecular markers, glycemic control, cardiovascular health, reduced blood pressure, and weight loss (intake of high-fiber foods) [16,132,184,185,186,187,188,189,190,191]. (3) However, this diet needs constant nutritional monitoring, because in the long term it can reduce intake of some micronutrients, such as iron, calcium, and vitamin B12 [16,132,184,185,186,187,188,189,190,191]. |
| Paleolithic Diet | The Paleolithic diet consists of following similar eating habits as our ancestors. It encourages the consumption of all types of meat, animal fat, fruits, vegetables, roots, raw foods and all types of food that nature can offer. The habit of fasting is also recommended in this type of dietary pattern [16,132,192,193,194]. | Low | (1) Low long-term adherence [16,132,192,193,194]. (2) It has become technically impossible to follow a diet identical to the Paleolithic period in Westernized society [16,132,192,193,194]. (3) More studies are needed in patients with Diabetes [16,132,192,193,194]. |
| Intermittent Fasting | Fasting means abstaining from foods and drinks that contain macronutrients and calories. Daily caloric intake occurs within a defined eating window during the day, and there are several types of fasting protocols (16 to 24 h), aiming to enhance the production of ketone bodies [16,132,195,196,197]. | Low | (1) Low long-term adherence [16,132,195,196,197]. (2) It can increase the risk of dehydration, headache, hypoglycemia, and lack of glycemic control [16,132,195,196,197]. (3) It is strongly discouraged by guidelines for patients with diabetes, mainly in diabetic ketoacidosis [16,132,195,196,197]. (4) More studies are needed in patients with diabetes [16,132,195,196,197]. |
| Mindful Eating | The practices of Mindful Eating can help increase knowledge of the factors (physiological, environmental, or emotional) that lead to excessive food consumption and training in intuitive eating (respecting signs of hunger/satiety and chewing food). The program not only values the quantity and quality consumed, but also the pleasure of the experience, thereby changing the value of the food reward without restricting it [16,132,198,199]. | Moderate | (1) Moderate long-term adherence [16,132,198,199]. (2) It is a type of nutritional strategy that has been gaining prominence in the literature with good results [16,132,198,199]. (3) However, more studies are needed to evaluate the impact of mindful eating on weight loss, glycemic control, and improvement in cardiovascular markers [16,132,198,199]. (4) Seems to be a good strategy to take care of the subjective issues of nutritional management [16,132,198,199]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minari, T.P.; Tácito, L.H.B.; Yugar, L.B.T.; Ferreira-Melo, S.E.; Manzano, C.F.; Pires, A.C.; Moreno, H.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Yugar-Toledo, J.C. Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review. Nutrients 2023, 15, 5096. https://doi.org/10.3390/nu15245096
Minari TP, Tácito LHB, Yugar LBT, Ferreira-Melo SE, Manzano CF, Pires AC, Moreno H, Vilela-Martin JF, Cosenso-Martin LN, Yugar-Toledo JC. Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review. Nutrients. 2023; 15(24):5096. https://doi.org/10.3390/nu15245096
Chicago/Turabian StyleMinari, Tatiana Palotta, Lúcia Helena Bonalume Tácito, Louise Buonalumi Tácito Yugar, Sílvia Elaine Ferreira-Melo, Carolina Freitas Manzano, Antônio Carlos Pires, Heitor Moreno, José Fernando Vilela-Martin, Luciana Neves Cosenso-Martin, and Juan Carlos Yugar-Toledo. 2023. "Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review" Nutrients 15, no. 24: 5096. https://doi.org/10.3390/nu15245096
APA StyleMinari, T. P., Tácito, L. H. B., Yugar, L. B. T., Ferreira-Melo, S. E., Manzano, C. F., Pires, A. C., Moreno, H., Vilela-Martin, J. F., Cosenso-Martin, L. N., & Yugar-Toledo, J. C. (2023). Nutritional Strategies for the Management of Type 2 Diabetes Mellitus: A Narrative Review. Nutrients, 15(24), 5096. https://doi.org/10.3390/nu15245096