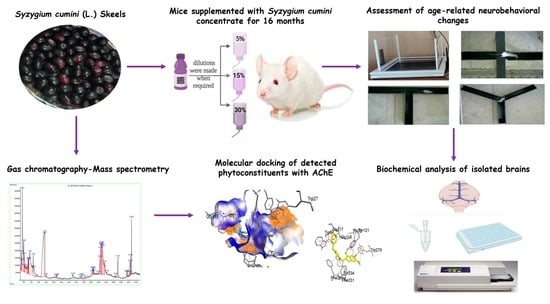
Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sy. cmi Fruit Dilution Preparation
2.2. Drugs and Chemicals
2.3. Animals and Their Housing
2.4. Animal Grouping and Their Diet
2.5. Behavioral Experiments
2.6. Total Phenols and Flavonoid Content Estimation
2.7. Gas Chromatography-Mass Spectrometry (GC-MS)
2.8. Behavioral Assessment for Locomotor and Anxiolytic Activity
2.8.1. Open Field Test (OFT)
2.8.2. Light-Dark (LD) Test
2.8.3. Elevated-Plus Maze (EPM) Test
2.9. Behavioral Tests for Learning and Memory
2.9.1. T-Maze (Spontaneous Alteration) Test
2.9.2. Y-Maze (Novel Arm) Test
2.9.3. Novel Object Recognition Test
2.9.4. Social Novelty Preference Test
2.9.5. Passive Avoidance (Step-Through) Test
2.9.6. Barnes Maze Test
2.10. Preparation of Brain Homogenates for Biochemical Analysis
2.10.1. Malondialdehyde (MDA) Assay
2.10.2. Superoxide Dismutase (SOD) Assay
2.10.3. Glutathione Peroxidase (GPx) Assay
2.10.4. Catalase (CAT) Assay
2.10.5. Acetylcholinesterase (AChE) Assay
2.11. Molecular Docking Methodology
2.11.1. Selection of Protein Targets
2.11.2. Softwares Required
2.11.3. Preparation of Protein
2.11.4. Preparation of Ligand and Molecular Docking
2.11.5. Visualization
2.11.6. Validation
2.12. Statistical Analysis
3. Results
3.1. Total Phenols and Flavonoids Content
3.2. GC–MS Analysis for Phytocomponents
3.3. Impact of Sy. cmi on Anxiety-like Behavior and Locomotor Activity in an Open Field
3.4. Impact of Sy. cmi on Anxiety-like Behavior in Light/Dark and ElevatedPlus-Maze Tests
3.5. Impact of Sy. cmi on Learning and Memory in T-Maze and Y-Maze Tests
3.6. Impact of Sy. cmi on Learning and Memory in Familiarity/Novelty Preference Tests
3.7. Impact of Sy. cmi on Learning and Memory in Passive Avoidance Test
3.8. Impact of Sy. cmi on Learning and Memory in Barnes Maze Test
3.9. Biochemical Analysis of Isolated Brains for MDA, SOD, GPx, Catalase, and AChE
3.10. Molecular Docking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gonzales, M.M.; Garbarino, V.R.; Pollet, E.; Palavicini, J.P.; Kellogg, D.L.; Kraig, E.; Orr, M.E. Biological aging processes underlying cognitive decline and neurodegenerative disease. J. Clin. Investig. 2022, 132, e158453. [Google Scholar] [CrossRef] [PubMed]
- Deture, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajmohan, R.; Reddy, P.H. Amyloid-beta and phosphorylated tau accumulations cause abnormalities at synapses of Alzheimer’s disease neurons. J. Alzheimer’s Dis. 2017, 57, 975–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrasek, T.; Skurlova, M.; Maleninska, K.; Vojtechova, I.; Kristofikova, Z.; Matuskova, H.; Sirova, J.; Vales, K.; Ripova, D.; Stuchlik, A. A rat model of Alzheimer’s disease based on Abeta42 and pro-oxidative substances exhibits cognitive deficit and alterations in glutamatergic and cholinergic neurotransmitter systems. Front. Aging Neurosci. 2016, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Van Dam, D.; Vermeiren, Y.; Dekker, A.D.; Naudé, P.J.W.; Deyn, P.P. De Neuropsychiatric disturbances in alzheimer’s disease: What have we learned from neuropathological studies? Curr. Alzheimer Res. 2016, 13, 1145–1164. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimers. Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef] [Green Version]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s disease:: Getting on and staying on. Curr. Ther. Res. Clin. Exp. 2003, 64, 216–235. [Google Scholar] [CrossRef] [Green Version]
- Milošević, M.; Arsić, A.; Cvetković, Z.; Vučić, V. Memorable Food: Fighting Age-Related Neurodegeneration by Precision Nutrition. Front. Nutr. 2021, 8, 688086. [Google Scholar] [CrossRef]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Onaolapo, A.Y.; Obelawo, A.Y.; Onaolapo, O.J. Brain ageing, cognition and diet: A review of the emerging roles of food-based nootropics in mitigating age-related memory decline. Curr. Aging. Sci. 2019, 12, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, N.; Dolatkhah, N.; Salekzamani, Y.; Hashemian, M. Effect of berry-based supplements and foods on cognitive function: A systematic review. Sci. Rep. 2022, 12, 3239. [Google Scholar] [CrossRef] [PubMed]
- Subash, S.; Essa, M.M.; Al-Adawi, S.; Memon, M.A.; Manivasagam, T.; Akbar, M. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen. Res. 2014, 9, 1557–1566. [Google Scholar] [PubMed]
- Kelly, E.; Vyas, P.; Weber, J.T. Biochemical Properties and Neuroprotective Effects of Compounds in Various Species of Berries. Molecules 2018, 23, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayyanar, M.; Subash-babu, P. Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pac. J. Trop. Biomed. 2012, 2, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Chandra, D. Pharmacological potentials of Syzygium cumini: A review. J. Sci. Food. Agric. 2013, 93, 2084–2093. [Google Scholar] [CrossRef]
- Stanely Mainzen Prince, P.; Kamalakkannan, N.; Menon, V.P. Syzigium cumini seed extracts reduce tissue damage in diabetic rat brain. J. Ethnopharmacol. 2003, 84, 205–209. [Google Scholar] [CrossRef]
- Alikatte, K.L.; Akondi, B.R.; Yerragunta, V.G.; Veerareddy, P.R.; Palle, S. Antiamnesic activity of Syzygium cumini against scopolamine induced spatial memory impairments in rats. Brain Dev. 2012, 34, 844–851. [Google Scholar] [CrossRef]
- Imran, I.; Javaid, S.; Waheed, A.; Rasool, M.F.; Majeed, A.; Samad, N.; Saeed, H.; Alqahtani, F.; Ahmed, M.M.; Alaqil, F.A. Grewia asiatica Berry Juice Diminishes Anxiety, Depression, and Scopolamine-Induced Learning and Memory Impairment in Behavioral Experimental Animal Models. Front. Nutr. 2021, 7, 587367. [Google Scholar] [CrossRef]
- Seibenhener, M.L.; Wooten, M.C. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin- Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Shoji, H. Age-related behavioral changes from young to old age in male mice of a C57BL/6J strain maintained under a genetic stability program. Neuropsychopharmacol. Rep. 2019, 39, 100–118. [Google Scholar] [CrossRef]
- Shakeel, W.; Javaid, S.; Rasool, M.F.; Samad, N.; Alasmari, F.; Alasmari, A.F.; Alaqil, F.A.; Alqarni, S.A.; Alotaibi, F.M.; Alqahtani, F.; et al. Time course evaluation of lacosamide alone and in polypharmacy on behavioral manifestations and oxidative stress in lithium-pilocarpine-induced model. J. Physiol. Pharmacol. 2020, 71, 547–564. [Google Scholar]
- Béracochéa, D. Interaction between diazepam and hippocampal corticosterone after acute stress: Impact on memory in middle-aged mice. Front. Behav. Neurosci. 2011, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Javaid, U.; Javaid, S.; Ashraf, W.; Rasool, M.F.; Noman, O.M.; Alqahtani, A.S.; Majeed, A.; Shakeel, W.; Albekairi, T.H.; Alqahtani, F.; et al. Chemical Profiling and Dose-Dependent Assessment of Fear Reducing and Memory-Enhancing Effects of Solanum virginianum in Rats. Dose. Response. 2021, 19, 1559325821998486. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J.; Rawlins, J.N.P. T-maze alternation in the rodent. Nat. Protoc. 2006, 1, 7–12. [Google Scholar] [CrossRef]
- Narayanan, R.; Gilbert, B.; Ravichandran, S.; Bhuvaneswari, K. Psychopharmacological characterization of effects of Ferula asafoetida linn. formulation in mouse on a Y-maze, EPM, and open field apparatus. J. Basic. Clin. Pharmacol. 2017, 6, 2254–2258. [Google Scholar] [CrossRef] [Green Version]
- Esquivel, N.; García, Y.; Lores, B.; Gutiérrez, M.; Rodríguez, C. Characterization of aged male BALB/c cenp mice as a model of dementia. BMC Genom. 2020, 36, 7. [Google Scholar]
- Rehman, Z.; Farooq, T.; Javaid, S.; Ashraf, W.; Fawad Rasool, M.; Samad, N.; Tariq, M.; Muhammad Muneeb Anjum, S.; Sivandzade, F.; Alotaibi, F.; et al. Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi Pharm. J. 2022, 30, 494–507. [Google Scholar] [CrossRef]
- Salomons, A.R.; Pinzon, N.E.; Boleij, H.; Kirchhoff, S.; Arndt, S.S.; Nordquist, R.E.; Lindemann, L.; Jaeschke, G.; Spooren, W.; Ohl, F. Differential effects of diazepam and MPEP on habituation and neuro-behavioural processes in inbred mice. Behav. Brain Funct. 2012, 8, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barzilay, R.; Sadan, O.; Bren, Z.; Taler, M.; Lev, N.; Tarasenko, I.; Uzan, R.; Melamed, E.; Weizman, A.; Offen, D. Intracerebral adult stem cells transplantation increases brain-derived neurotrophic factor levels and protects against phencyclidine-induced social deficit in mice. Transl. Psychiatry 2014, 1, e61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajjad Haider, M.; Ashraf, W.; Javaid, S.; Fawad Rasool, M.; Muhammad Abdur Rahman, H.; Saleem, H.; Muhammad Muneeb Anjum, S.; Siddique, F.; Morales-Bayuelo, A.; Kaya, S.; et al. Chemical characterization and evaluation of the neuroprotective potential of Indigofera sessiliflora through in-silico studies and behavioral tests in scopolamine-induced memory compromised rats. Saudi J. Biol. Sci. 2021, 28, 4384–4398. [Google Scholar] [CrossRef] [PubMed]
- Gawel, K. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn. Schmiedebergs. Arch. Pharmacol. 2019, 392, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio. Protoc. 2007, 8, e2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waterborg, J.H.; Matthew, H.R. The Lowry method for protein quantitation. Methods Mol. Biol. 1984, 1, 7–10. [Google Scholar]
- Singh, R.P.; Chidambara Murthy, K.N.; Jayaprakasha, G.K. Studies on the antioxidant activity of pomegranate (Punica granatum) peel and seed extracts using in vitro models. J. Agric. Food Chem. 2002, 50, 81–86. [Google Scholar] [CrossRef]
- Haider, S.; Naqvi, F.; Batool, Z.; Tabassum, S.; Sadir, S. Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male rats Pretreatment with curcumin attenuates anxiety while strengthens memory performance after one short stress experience in male. Brain Res. Bull. 2015, 115, 1–8. [Google Scholar] [CrossRef]
- Farjad, E.; Momeni, H.R. Silymarin ameliorates oxidative stress and enhances antioxidant defense system capacity in cadmium-treated mice. Cell J. Yakhteh 2018, 20, 422–426. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- RCSB PDB-1GQR. Available online: https://www.rcsb.org/structure/1GQR (accessed on 6 January 2023).
- Morris, G.M.; Ruth, H.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Software news and updates AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Accelrys Software Inc. Discovery Studio Visualizer, 2; Accelrys Software Inc.: San Diego, CA, USA, 2005. [Google Scholar]
- CambridgeSoft. ChemDraw Ultra 12.0 0 (Copyright) 1986 to 2009; CambridgeSoft Corp.: Cambridge, MA, USA, 2009. [Google Scholar]
- CambridgeSoft. Chem 3D Pro 12.0 (Copyright) 1986 to 2009; CambridgeSoft Corp.: Cambridge, MA, USA, 2009. [Google Scholar]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zentgraf, M.; Steuber, H.; Koch, C.; La Motta, C.; Sartini, S.; Sotriffer, C.A.; Klebe, G. How reliable are current docking approaches for structure-based drug design? Lessons from aldose reductase. Angew. Chem. Int. Ed. Engl. 2007, 46, 3575–3578. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, D.; Davis, A.M.; Kleywegt, G.J.; Schmitt, S. An alternative method for the evaluation of docking performance: RSR vs RMSD. J. Chem. Inf. Model. 2008, 48, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Selvi, S.; Polat, R.; Çakilcioğlu, U.; Celep, F.; Dirmenci, T.; Ertuğ, Z.F. An ethnobotanical review on medicinal plants of the Lamiaceae family in Turkey. Turk. J. Botany 2022, 46, 283–332. [Google Scholar] [CrossRef]
- Çakılcıoğlu, U. An ethnobotanical field study; traditional foods production and medicinal utilization of Gundelia L. species in Tunceli (Turkey). Indian J. Tradit. Knowl. 2020, 19, 714–718. [Google Scholar]
- Sarkar, S.M.; Dhar, B.K.; Crowley, S.S.; Ayittey, F.K.; Gazi, M.A.I. Psychological adjustment and guidance for ageing urban women. Ageing Int. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Cole, G.; Head, E.; Ingram, D. Nutrition, brain aging, and neurodegeneration. J. Neurosci. 2009, 29, 12795–12801. [Google Scholar] [CrossRef] [Green Version]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Swallah, M.S.; Sun, H.; Affoh, R.; Fu, H.; Yu, H. Antioxidant potential overviews of secondary metabolites (polyphenols) in fruits. Int. J. Food Sci. 2020, 2020, 9081686. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive polyphenols and neuromodulation: Molecular mechanisms in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 2564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolte, E.D.; Nolte, K.A.; Yan, S.S. Anxiety and task performance changes in an aging mouse model. Biochem. Biophys. Res. Commun. 2019, 514, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Ríos, J.L.; Schinella, G.R.; Moragrega, I. Phenolics as GABAA Receptor Ligands: An Updated Review. Molecules 2022, 27, 1770. [Google Scholar] [CrossRef]
- Tomić, M.; Ignjatović, D.D.; Tovilović-Kovačević, G.; Krstić-Milošević, D.; Ranković, S.; Popović, T.; Glibetić, M. Reduction of anxiety-like and depression-like behaviors in rats after one month of drinking: Aronia melanocarpa berry juice. Food Funct. 2016, 7, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Qin, T.; Sun, D.; Zhao, X.; Zhang, B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav. 2020, 10, e01633. [Google Scholar] [CrossRef] [PubMed]
- Rees, T.M.; Brimijoin, S. The role of acetylcholinesterase in the pathogenesis of Alzheimer’s disease. Drugs Today 2003, 39, 75–83. [Google Scholar] [CrossRef]
- Hussain, G.; Zhang, L.; Rasul, A.; Anwar, H.; Sohail, M.U.; Razzaq, A.; Aziz, N.; Shabbir, A.; Ali, M.; Sun, T. Role of plant-derived flavonoids and their mechanism in attenuation of Alzheimer’s and Parkinson’s diseases: An update of recent data. Molecules 2018, 23, 814. [Google Scholar] [CrossRef] [Green Version]
- Dias, G.P.; Cavegn, N.; Nix, A.; Do Nascimento Bevilaqua, M.C.; Stangl, D.; Zainuddin, M.S.A.; Nardi, A.E.; Gardino, P.F.; Thuret, S. The role of dietary polyphenols on adult hippocampal neurogenesis: Molecular mechanisms and behavioural effects on depression and anxiety. Oxid. Med. Cell. Longev. 2012, 2012, 541971. [Google Scholar] [CrossRef] [Green Version]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2020, 154, 35–44. [Google Scholar] [CrossRef]
- Bensalem, J.; Dudonné, S.; Gaudout, D.; Servant, L.; Calon, F.; Desjardins, Y.; Layé, S.; Lafenetre, P.; Pallet, V. Polyphenol-rich extract from grape and blueberry attenuates cognitive decline and improves neuronal function in aged mice. J. Nutr. Sci. 2018, 7, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, L.P.; Daphney, C.M.; Oppong-Damoah, A.; Uchakin, P.N.; Abney, S.E.; Uchakina, O.N.; Khusial, R.D.; Akil, A.; Murnane, K.S. The cannabinoid receptor 2 agonist, β-caryophyllene, improves working memory and reduces circulating levels of specific proinflammatory cytokines in aged male mice. Behav. Brain Res. 2019, 372, 112012. [Google Scholar] [CrossRef]
- Sudeep, H.V.; Venkatakrishna, K.; Amritharaj; Gouthamchandra, K.; Reethi, B.; Naveen, P.; Lingaraju, H.B.; Shyamprasad, K. A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins. J. Food Biochem. 2021, 45, e13994. [Google Scholar] [CrossRef]
- Ojha, S.; Javed, H.; Azimullah, S.; Haque, M.E. β-Caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol. Cell. Biochem. 2016, 418, 59–70. [Google Scholar] [CrossRef]
- Wu, J.; Bie, B.; Yang, H.; Xu, J.J.; Brown, D.L.; Naguib, M. Activation of the CB2 receptor system reverses amyloid-induced memory deficiency. Neurobiol. Aging 2013, 34, 791–804. [Google Scholar] [CrossRef]
- Aso, E.; Ferrer, I. CB2 Cannabinoid receptor as potential target against Alzheimer’s disease. Front. Neurosci. 2016, 10, 243. [Google Scholar] [CrossRef] [Green Version]
- Arslan, M.E.; Türkez, H.; Mardinoğlu, A. In vitro neuroprotective effects of farnesene sesquiterpene on alzheimer’s disease model of differentiated neuroblastoma cell line. Int. J. Neurosci. 2021, 131, 745–754. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory potential of various sesquiterpene analogues for Alzheimer’s disease therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Sathya, S.; Manogari, B.G.; Thamaraiselvi, K.; Vaidevi, S.; Ruckmani, K.; Devi, K.P. Phytol loaded PLGA nanoparticles ameliorate scopolamine-induced cognitive dysfunction by attenuating cholinesterase activity, oxidative stress and apoptosis in Wistar rat. Nutr. Neurosci. 2020, 25, 485–501. [Google Scholar] [CrossRef] [PubMed]













| Serial No. | Phytocompounds | RT (Retention Time) | Area% |
|---|---|---|---|
| 1 | trans-beta-Ocimene | 2.271 | 0.37 |
| 2 | 5-Hydroxymethylfurfural | 7.449 | 18.76 |
| 3 | Caryophyllene | 15.098 | 0.80 |
| 4 | Humulene | 17.076 | 0.38 |
| 5 | beta-Farnesene | 17.508 | 0.43 |
| 6 | Dodecanoic acid | 20.579 | 0.46 |
| 7 | Oleic Acid | 30.500 | 1.13 |
| 8 | Phytol | 30.139 | 0.18 |
| Ligands with 1GQR | Binding Affinity, ΔG (kcal/mol) | Hydrogen-Binding Interaction, Residue (Distance Å) | Hydrophobic Interaction, Residue (Distance Å) | Electrostatic Interaction, Residue (Distance Å) |
|---|---|---|---|---|
| Caryophyllene | −8.1 | TYR121(2.89) GLY118(2.79) TRP84(2.63) SER122(3.01) | TYR334(3.96) PHE331(3.65) ASP72(4.30) ASN85(4.41) | PHE330(4.29) |
| Humulene | −8.3 | PHE290(2.73) TYR121(2.97) GLY335(3.93) | TRP279(3.54) PHE331(3.64) ILE287(4.56) PHE288(4.89) | TYR334(4.12) |
| beta-Farnesene | −7.3 | HIS440(3.56) TRP84(4.12) TYR334(4.19) | PHE330(4.81) PHE331(4.46) | |
| Phytol | −6.6 | TRP279(3.63) TYR334(3.93) TRP84(3.72) | PHE330(4.26) PHE331(4.14) TYR70(4.73) HIS440(5.01) | |
| Donepezil | −10.4 | GLY118(2.38) | PHE330(3.96) TRP84(3.63) TYR70(4.98) TRP279(4.57) TYR121(4.11) TRP84(4.72) | TRP279(5.36) GLY117(5.82) PHE331(5.23) TYR334 (5.21) TRP279(5.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, N.; Javaid, S.; Ashraf, W.; Siddique, F.; Rasool, M.F.; Alqahtani, F.; Ahmad, T.; Abrar, M.A.; Imran, I. Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice. Nutrients 2023, 15, 666. https://doi.org/10.3390/nu15030666
Malik N, Javaid S, Ashraf W, Siddique F, Rasool MF, Alqahtani F, Ahmad T, Abrar MA, Imran I. Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice. Nutrients. 2023; 15(3):666. https://doi.org/10.3390/nu15030666
Chicago/Turabian StyleMalik, Nosheen, Sana Javaid, Waseem Ashraf, Farhan Siddique, Muhammad Fawad Rasool, Faleh Alqahtani, Tanveer Ahmad, Muhammad Asad Abrar, and Imran Imran. 2023. "Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice" Nutrients 15, no. 3: 666. https://doi.org/10.3390/nu15030666
APA StyleMalik, N., Javaid, S., Ashraf, W., Siddique, F., Rasool, M. F., Alqahtani, F., Ahmad, T., Abrar, M. A., & Imran, I. (2023). Long-Term Supplementation of Syzygium cumini (L.) Skeels Concentrate Alleviates Age-Related Cognitive Deficit and Oxidative Damage: A Comparative Study of Young vs. Old Mice. Nutrients, 15(3), 666. https://doi.org/10.3390/nu15030666