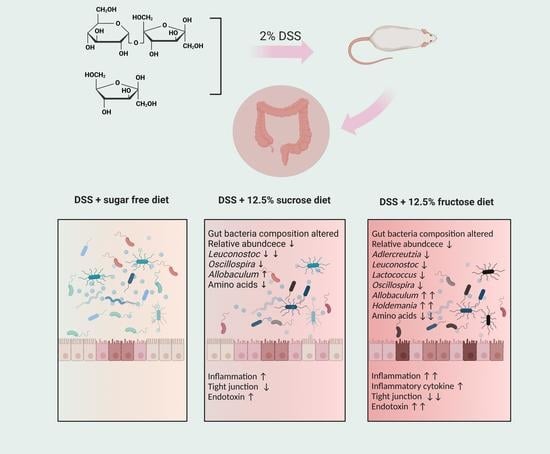
Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets and Animal Experimental Design
2.2. Histological Analysis
2.3. Oxidative Stress and Endotoxin Determination in Serum
2.4. Tight Junction Protein Expression Determination
2.5. Gut Microbacteria Analysis
2.6. Metabolomics Analysis
3. Results
3.1. Fructose and Sucrose Enhanced DSS-Induced Phenotypes
3.2. Fructose Induced Oxidative Stress and Inflammation in Serum
3.3. Sucrose and Fructose Altered the Overall Structure and Composition of Gut Microbiota in Rats
3.4. Sucrose and Fructose Altered Colonic Content Metabolites Profiles in Experimental Rats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almatroodi, S.A.; Anwar, S.; Almatroudi, A.; Khan, A.A.; Alrumaihi, F.; Alsahli, M.A.; Rahmani, A.H. Hepatoprotec-tive Effects of Garlic Extract against Carbon Tetrachloride (CCl4)-Induced Liver Injury via Modulation of Antioxi-dant, Anti-Inflammatory Activities and Hepatocyte Architecture. Appl. Sci. 2020, 10, 6200. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and Disorders of the Stress System. Nat. Rev. Endocrinol. 2009, 5, 374–381. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Adams, L.A.; de Lédinghen, V.; Wong, G.L.-H.; Sookoian, S. Noninvasive Biomarkers in NAFLD and NAS—Current Progress and Future Promise. Nat. Rev. Gastroenterol.Hepatol. 2018, 15, 461–478. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The Gut-Liver Axis in Liver Disease: Pathophysiological Basis for Therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Karin, M. “Sweet Death”: Fructose as a Metabolic Toxin That Targets the Gut-Liver Axis. Cell Metab. 2021, 33, 2316–2328. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular Aspects of Fructose Metabolism and Metabolic Disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF- B Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Saengboonmee, C.; Phoomak, C.; Supabphol, S.; Covington, K.R.; Hampton, O.; Wongkham, C.; Gibbs, R.A.; Umezawa, K.; Seubwai, W.; Gingras, M.-C.; et al. NF-ΚB and STAT3 Co-Operation Enhances High Glucose Induced Aggressiveness of Cholangiocarcinoma Cells. Life Sci. 2020, 262, 118548. [Google Scholar] [CrossRef]
- Cho, Y.-E.; Kim, D.-K.; Seo, W.; Gao, B.; Yoo, S.-H.; Song, B.-J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef]
- Khan, S.; Waliullah, S.; Godfrey, V.; Khan, M.A.W.; Ramachandran, R.A.; Cantarel, B.L.; Behrendt, C.; Peng, L.; Hooper, L.V.; Zaki, H. Dietary Simple Sugars Alter Microbial Ecology in the Gut and Promote Colitis in Mice. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Montrose, D.C.; Nishiguchi, R.; Basu, S.; Staab, H.A.; Zhou, X.K.; Wang, H.; Meng, L.; Johncilla, M.; Cubillos-Ruiz, J.R.; Morales, D.K.; et al. Dietary Fructose Alters the Composition, Localization, and Metabolism of Gut Microbiota in Association With Worsening Colitis. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 525–550. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, W.; Song, G.; Pang, S.; Peng, Z.; Li, Y.; Wang, P. High-Fructose Diet Increases Inflammatory Cytokines and Alters Gut Microbiota Composition in Rats. Mediat. Inflamm. 2020, 2020, 6672636. [Google Scholar] [CrossRef]
- Szlas, A.; Kurek, J.M.; Krejpcio, Z. The Potential of L-Arginine in Prevention and Treatment of Disturbed Carbohydrate and Lipid Metabolism—A Review. Nutrients 2022, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef]
- Anwar, S.; Almatroudi, A.; Allemailem, K.S.; Jacob Joseph, R.; Khan, A.A.; Rahmani, A.H. Protective Effects of Ginger Extract against Glycation and Oxidative Stress-Induced Health Complications: An In Vitro Study. Processes 2020, 8, 468. [Google Scholar] [CrossRef]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant Gut Microbiota Alters Host Metabolome and Impacts Renal Failure in Humans and Rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A Simple Practice Guide for Dose Conversion between Animals and Human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Y.; Gao, Y.; Zhang, P.; Huang, W.; Liu, Y.; Huang, Y.; Tian, Y.; Wan, J.-B.; Zhang, Z.; et al. An Extendable All-in-One Injection Twin Derivatization LC-MS/MS Strategy for the Absolute Quantification of Multiple Chemical-Group-Based Submetabolomes. Anal. Chim. Acta 2019, 1063, 99–109. [Google Scholar] [CrossRef]
- Jian, X.; Zhu, Y.; Ouyang, J.; Wang, Y.; Lei, Q.; Xia, J.; Guan, Y.; Zhang, J.; Guo, J.; He, Y.; et al. Alterations of Gut Microbiome Accelerate Multiple Myeloma Progression by Increasing the Relative Abundances of Nitrogen-Recycling Bacteria. Microbiome 2020, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Softic, S.; Stanhope, K.L.; Boucher, J.; Divanovic, S.; Lanaspa, M.A.; Johnson, R.J.; Kahn, C.R. Fructose and Hepatic Insulin Resistance. Crit. Rev. Clin. Lab. Sci 2020, 57, 308–322. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary Intake and Risk of Developing Inflammatory Bowel Disease: A Systematic Review of the Literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.M.; Luben, R.; van Schaik, F.; Oldenburg, B.; Bueno-de-Mesquita, H.B.; Hallmans, G.; Karling, P.; Lindgren, S.; Grip, O.; Key, T.; et al. Carbohydrate Intake in the Etiology of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2014, 20, 2013–2021. [Google Scholar] [CrossRef]
- Persson, P.G.; Ahlbom, A.; Hellers, G. Diet and Inflammatory Bowel Disease: A Case-Control Study. Epidemiology 1992, 3, 47–52. [Google Scholar] [CrossRef]
- Galipeau, H.J.; Caminero, A.; Turpin, W.; Bermudez-Brito, M.; Santiago, A.; Libertucci, J.; Constante, M.; Raygoza Garay, J.A.; Rueda, G.; Armstrong, S.; et al. Novel Fecal Biomarkers That Precede Clinical Diagnosis of Ulcerative Colitis. Gastroenterology 2021, 160, 1532–1545. [Google Scholar] [CrossRef]
- Tamana, S.K.; Tun, H.M.; Konya, T.; Chari, R.S.; Field, C.J.; Guttman, D.S.; Becker, A.B.; Moraes, T.J.; Turvey, S.E.; Subbarao, P.; et al. Bacteroides-Dominant Gut Microbiome of Late Infancy Is Associated with Enhanced Neurodevelopment. Gut Microbes 2021, 13, 1930875. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.; Hou, Y.; Zhang, X.; Yin, Z.; Cai, X.; Wei, W.; Wang, J.; He, D.; Wang, G.; et al. Bacteroides Species Differentially Modulate Depression-like Behavior via Gut-Brain Metabolic Signaling. Brain Behav. Immun. 2022, 102, 11–22. [Google Scholar] [CrossRef]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in Health and Disease, a Driver or Just along for the Ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Schifano, E.; Tomassini, A.; Preziosi, A.; Montes, J.; Aureli, W.; Mancini, P.; Miccheli, A.; Uccelletti, D. Leuconostoc mesenteroides Strains Isolated from Carrots Show Probiotic Features. Microorganisms 2021, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.-L.; Yang, Y.-L.; Zhang, Z.; Yao, Y.-Y.; Xia, R.; Gao, C.-C.; Du, D.-D.; Hu, J.; Ran, C.; Liu, Z.; et al. Surface-Displayed Amuc_1100 From on ZHY1 Improves Hepatic Steatosis and Intestinal Health in High-Fat-Fed Zebrafish. Front. Nutr. 2021, 8, 726108. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut Microbiome and Health: Mechanistic Insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, Q.; Balakrishnan, B.; Belobrajdic, D.P.; Feng, Q.-J.; Zhang, W. Role of Dietary Nutrients in the Modulation of Gut Microbiota: A Narrative Review. Nutrients 2020, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, U.; Akdis, M.; Akdis, C.A. 1—Introduction to Mechanisms of Allergic Diseases. In Allergy Essentials, 2nd ed.; O’Hehir, R.E., Holgate, S.T., Khurana Hershey, G.K., Sheikh, A., Eds.; Elsevier: Philadelphia, PA, USA, 2022; pp. 1–24. ISBN 978-0-323-80912-2. [Google Scholar]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272.e6. [Google Scholar] [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front. Cell. Infect. Microbiol. 2021, 11, 757718. [Google Scholar] [CrossRef]
- Jeong, Y.; Jhun, J.; Lee, S.-Y.; Na, H.S.; Choi, J.; Cho, K.-H.; Lee, S.Y.; Lee, A.R.; Park, S.-J.; You, H.J.; et al. Therapeutic Potential of a Novel Identified Through Microbiome Profiling of RA Patients With Different RF Levels. Front. Immunol. 2021, 12, 736196. [Google Scholar] [CrossRef] [PubMed]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A Novel Ruminococcus Gnavus Clade Enriched in Inflammatory Bowel Disease Patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Wu, F.; Davey, S.; Clendenen, T.V.; Koenig, K.L.; Afanasyeva, Y.; Zhou, B.; Bedi, S.; Li, H.; Zeleniuch-Jacquotte, A.; Chen, Y. Gut Microbiota and Subjective Memory Complaints in Older Women. J. Alzheimers Dis. 2022, 88, 251–262. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.A.; Bielecka, A.A.; Nguyen, M.T.; Rosen, C.E.; Song, D.; Sonnert, N.D.; Yang, Y.; Cao, Y.; Khetrapal, V.; Catanzaro, J.R.; et al. Interspecies Commensal Interactions Have Nonlinear Impacts on Host Immunity. Cell Host Microbe 2022, 30. [Google Scholar] [CrossRef] [PubMed]
- Heilbronner, S.; Foster, T.J. Staphylococcus lugdunensis: A Skin Commensal with Invasive Pathogenic Potential. Clin. Microbiol. Rev. 2021, 34, e00205-20. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The Gut Microbiota in Anxiety and Depression—A Systematic Review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.M.; Hennon, S.W.; Feldman, M.F. Uncovering the Mechanisms of Acinetobacter baumannii Virulence. Nat. Rev. Microbiol. 2018, 16, 91–102. [Google Scholar] [CrossRef]
- Bonwitt, J.; Tran, M.; Droz, A.; Gonzalez, A.; Glover, W.A. Psychrobacter sanguinis Wound Infection Associated with Marine Environment Exposure, Washington, USA. Emerg. Infect. Dis. 2018, 24, 1942–1944. [Google Scholar] [CrossRef]
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. Infections. Nat. Rev. Dis. Prim. 2018, 4, 1–19. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.J.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.F.P.M.; Baade-Corpelijn, L.; et al. Gut Microbiota and Short-Chain Fatty Acid Alterations in Cachectic Cancer Patients. J. Cachexia Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef]
- Kelly, B.; Pearce, E.L. Amino Assets: How Amino Acids Support Immunity. Cell Metab. 2020, 32, 154–175. [Google Scholar] [CrossRef]
- Jones, N.; Blagih, J.; Zani, F.; Rees, A.; Hill, D.G.; Jenkins, B.J.; Bull, C.J.; Moreira, D.; Bantan, A.I.M.; Cronin, J.G.; et al. Fructose Reprogrammes Glutamine-Dependent Oxidative Metabolism to Support LPS-Induced Inflammation. Nat. Commun. 2021, 12, 1209. [Google Scholar] [CrossRef] [PubMed]







| No. | Pathway | Hits | Raw P | Impact | Enriched Compounds |
|---|---|---|---|---|---|
| 1 | Aminoacyl-tRNA biosynthesis | 16 | 1.15 × 10−9 | 0.35412 | Histidine (↓) Phenylalanine (↓) Arginine (↓) Glycine (↓) Aspartic acid (↓) Serine (↓) Methionine (↓) Valine (↓) Alanine (↓) Lysine (↓) Isoleucine (↓) Leucine (↓) Threonine (↓) Tryptophan (↓) Tyrosine (↓) Proline (↓) |
| 2 | Arginine and proline metabolism | 6 | 0.006732 | 0.24491 | Citrulline Aspartic acid (↓) Arginine (↓) Proline (↓) 4-Hydroxyproline (↓) Gamma amino butyric acid (GABA) (↓) |
| 3 | Valine, leucine and isoleucine biosynthesis | 3 | 0.007934 | 0.57143 | Leucine (↓) Valine (↓) Isoleucine (↓) |
| 4 | Phenylalanine, tyrosine and tryptophan biosynthesis | 2 | 0.008931 | 0.75 | Phenylalanine (↓) Tyrosine (↓) |
| 5 | Cyanoamino acid metabolism | 2 | 0.021203 | 0.33333 | Glycine (↓) Serine (↓) |
| 6 | Nitrogen metabolism | 2 | 0.047114 | 0.11111 | Histidine (↓) Glycine (↓) |
| 7 | Methane metabolism | 2 | 0.047114 | 0.16667 | Glycine (↓) Serine (↓) |
| 8 | Phenylalanine metabolism | 2 | 0.047114 | 0.44444 | Phenylalanine (↓) Tyrosine (↓) |
| Potential Biological Effect | Potential Function | References | ||
|---|---|---|---|---|
| 1 | Adlercreutzia | Beneficial | Ulcerative colitis biomarkers | [27] |
| 2 | Bacteroides | Beneficial/harmful | Anti-inflammatory/pro-inflammatory | [28,29] |
| 3 | Melissococcus | NA | ||
| 4 | Lactobacillus | Beneficial | Improve immunity | [30] |
| 5 | Leuconostoc | Beneficial | Improve immunity | [31] |
| 6 | Lactococcus | Beneficial | Improve immunity | [32] |
| 7 | Anaerofustis | NA | ||
| 8 | (Pseudoramibacter) Eubacterium | Harmful | Pro-inflammatory | [33,34] |
| 9 | (Clostridiaceae) Clostridium | Harmful | Pro-inflammatory Infectious | [35,36] |
| 10 | Roseburia | Beneficial | Anti-inflammatory Improve immunity | [37] |
| 11 | (Ruminococcaceae) Clostridium | Harmful | Pro-inflammatory Infectious | [38] |
| 12 | (Ruminococcaceae) Ruminococcus | Beneficial | Anti-inflammatory/pro-inflammatory | [34,39,40] |
| 13 | Faecalibaterium | Beneficial | Fatty-acid-producing bacteria | [41] |
| 14 | Oscillospira | Beneficial | Butyrate-producing-bacteria | [42] |
| 15 | (Erysipelotrichaceae) Eubacterium | Harmful | Pro-inflammatory | [33,34] |
| 16 | Allobaculum | Harmful | Induce colitis | [43] |
| 17 | Coprobacillus | Harmful | Proinflammatory | [39] |
| 18 | Holdemania | Harmful | Anxiety | [41] |
| 19 | Paracoccus | NA | ||
| 20 | (Lachnospiraceae) Clostridium | Harmful | Pro-inflammatory Infectious | [33,39] |
| 21 | Staphylococcus | Harmful | Infectious | [44] |
| 22 | Jeotgalicoccus | NA | ||
| 23 | Sutterella | Pathogen | Diarrhea | [45] |
| 24 | Desulfovibrio | Pathogen | H2S producing | [46] |
| 25 | Acinetobacter | Pathogen | Infectious | [47] |
| 26 | Psychrobacter | NA | [48] | |
| 27 | Vibrio | Pathogen | Infectious | [49] |
| 28 | Veillonella | Beneficial | Inhibit toxic bile acids | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, G.; Gan, Q.; Qi, W.; Wang, Y.; Xu, M.; Li, Y. Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats. Nutrients 2023, 15, 782. https://doi.org/10.3390/nu15030782
Song G, Gan Q, Qi W, Wang Y, Xu M, Li Y. Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats. Nutrients. 2023; 15(3):782. https://doi.org/10.3390/nu15030782
Chicago/Turabian StyleSong, Ge, Qianyun Gan, Wentao Qi, Yong Wang, Meihong Xu, and Yong Li. 2023. "Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats" Nutrients 15, no. 3: 782. https://doi.org/10.3390/nu15030782
APA StyleSong, G., Gan, Q., Qi, W., Wang, Y., Xu, M., & Li, Y. (2023). Fructose Stimulated Colonic Arginine and Proline Metabolism Dysbiosis, Altered Microbiota and Aggravated Intestinal Barrier Dysfunction in DSS-Induced Colitis Rats. Nutrients, 15(3), 782. https://doi.org/10.3390/nu15030782