Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review
Abstract
:1. Introduction
2. Materials and Methods
3. Cardiovascular Disease Risk Factors in SLE
3.1. Traditional Risk Factors in SLE Patients
3.1.1. Obesity
3.1.2. Dyslipidemia
3.1.3. Insulin Resistance
3.1.4. Smoking
3.1.5. Hypertension
3.1.6. Sedentary Lifestyle
3.1.7. MetS
3.2. SLE Non-Traditional Risk Factors
3.2.1. Hyperhomocysteinemia
3.2.2. Antiphospholipid Antibodies and Complex Immune Damage
3.2.3. Pro-Inflammatory Cytokines
3.2.4. C-Reactive Protein
3.2.5. Lupus Nephritis
3.2.6. SLE Pharmacotherapy Associated with Increased CVD Risk
Glucocorticoids
Methotrexate
3.2.7. SLE Pharmacotherapy Associated with Decreased CVD Risk
Antimalarials
Mycophenolate Mofetil
4. Potential Therapeutic Effect of Nutrients in SLE
4.1. Energy (Calories)
4.2. Polyunsaturated Fatty Acids, PUFA (n-3 and n-6)
4.3. Vitamin A
4.4. Antioxidant Vitamins (C and E)
4.5. B Vitamins
4.6. Vitamin D
4.7. Selenium
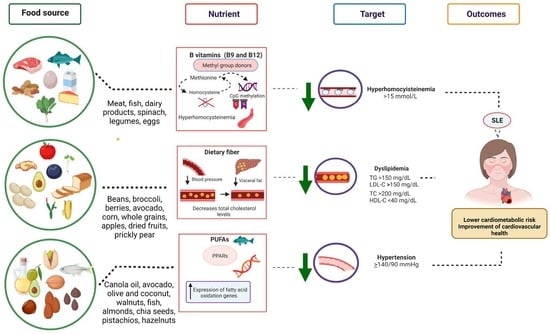
5. Nutrients to Target Cardiovascular Disease Risk in SLE Patients
5.1. PUFAs
5.2. Antioxidant Vitamins (C and E)
5.3. B Vitamins
5.4. Coenzyme Q10
5.5. Probiotics
5.6. Dietary Fiber
5.7. Vitamin A
5.8. Selenium
6. Limitations and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SLE | Systemic lupus erythematosus |
| CVD | cardiovascular disease |
| CRP | C-reactive protein |
| Hcy | homocysteine |
| IR | insulin resistance |
| Anti-oxLDL | antibodies against oxidized LDL |
| MetS | Metabolic syndrome |
| SLEDAI | Systemic Lupus Erythematosus Disease Activity Index |
| ADMA | Asymmetric dimethylarginine |
| MTHFR | Methylene tetrahydrofolate reductase |
| aPL | Antiphospholipid |
| IFNs | interferons |
| NF-κβ | Nuclear factor kappa beta |
| HCQ | Hydroxychloroquine |
| CQ | Chloroquine |
| MMF | Mycophenolate mofetil |
| PUFA | Polyunsaturated fatty acid |
| CoQ10 | Coenzyme Q10 |
| NO | nitric oxide |
References
- Ye, Y.; Wu, T.; Zhang, T.; Han, J.; Habazi, D.; Saxena, R.; Mohan, C. Elevated oxidized lipids, anti-lipid autoantibodies and oxidized lipid immune complexes in active SLE. Clin. Immunol. 2019, 205, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sinicato, N.A. Risk Factors in Cardiovascular Disease in Systemic Lupus Erythematosus. Curr. Cardiol. Rev. 2013, 9, 15–19. [Google Scholar] [PubMed] [Green Version]
- Wigren, M.; Nilsson, J.; Kaplan, M.J. Pathogenic immunity in systemic lupus erythematosus and atherosclerosis: Common mechanisms and possible targets for intervention. J. Intern. Med. 2015, 278, 494–506. [Google Scholar] [CrossRef] [Green Version]
- Croca, S.; Rahman, A. Atherosclerosis in systemic lupus erythematosus. Best Pract Res. Clin. Rheumatol. 2017, 31, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Meza-Meza, M.R.; Vizmanos-Lamotte, B.; Muñoz-Valle, J.F.; Parra-Rojas, I.; Garaulet, M.; Campos-López, B.; Montoya-Buelna, M.; Cerpa-Cruz, S.; Martínez-López, E.; Oregon-Romero, E.; et al. Relationship of Excess Weight with Clinical Activity and Dietary Intake Deficiencies in Systemic Lupus Erythematosus Patients. Nutrients 2019, 11, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hak, A.E.; Karlson, E.W.; Feskanich, D.; Stampfer, M.J.; Costenbader, K.H. Systemic lupus erythematosus and the risk of cardiovascular disease: Results from the nurses’ health study. Arthritis Rheum. 2009, 61, 1396–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzi, S.; Meilahn, E.N.; Rairie, J.E.; Conte, C.G.; Medsger, T.A.; Jansen-McWilliams, L.; D’Agostino, R.B.; Kuller, L.H. Age-specific Incidence Rates of Myocardial Infarction and Angina in Women with Systemic Lupus Erythematosus: Comparison with the Framingham Study. Am. J. Epidemiol. 1997, 145, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karp, I.; Abrahamowicz, M.; Fortin, P.R.; Pilote, L.; Neville, C.; Pineau, C.A. Esdaile JM. Recent corticosteroid use and recent disease activity: Independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum. 2008, 59, 169–175. [Google Scholar] [CrossRef]
- Szabó, M.Z.; Szodoray, P.; Kiss, E. Dyslipidemia in systemic lupus erythematosus. Immunol. Res. 2017, 65, 543–550. [Google Scholar] [CrossRef]
- Campos-López, B.; Meza-Meza, M.R.; Parra-Rojas, I.; Ruiz-Ballesteros, A.I.; Vizmanos-Lamotte, B.; Muñoz-Valle, J.F.; Montoya-Buelna, M.; Cerpa-Cruz, S.; Bernal-Hernández, L.E.; De la Cruz-Mosso, U. Association of cardiometabolic risk status with clinical activity and damage in systemic lupus erythematosus patients: A cross-sectional study. Clin. Immunol. 2021, 222, 108637. [Google Scholar] [CrossRef]
- de Miranda Moura dos Santos, F.; Borges, M.C.; Telles, R.W.; Correia, M.I.T.D.; Lanna, C.C.D. Excess weight and associated risk factors in patients with systemic lupus erythematosus. Rheumatol. Int. 2013, 33, 681–688. [Google Scholar] [CrossRef] [PubMed]
- La Cava, A. The Influence of Diet and Obesity on Gene Expression in SLE. Genes 2019, 10, 405. [Google Scholar] [CrossRef] [Green Version]
- Svenungsson, E.; Gunnarsson, I.; Fei, G.Z.; Lundberg, I.E.; Klareskog, L.; Frostegård, J. Elevated triglycerides and low levels of high-density lipoprotein as markers of disease activity in association with up-regulation of the tumor necrosis factor α/tumor necrosis factor receptor system in systemic lupus erythematosus: Blood Lipids and TNFα Activity in SLE. Arthritis Rheum. 2003, 48, 2533–2540. [Google Scholar]
- Oeser, A.; Chung, C.P.; Asanuma, Y.; Avalos, I.; Stein, C.M. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum. 2005, 52, 3651–3659. [Google Scholar] [CrossRef] [PubMed]
- Klack, K.; Bonfa, E.; Neto, E.F.B. Diet and nutritional aspects in systemic lupus erythematosus. Rev. Bras. Reumatol. 2012, 52, 384–408. [Google Scholar] [PubMed]
- Muthukumar, A.; Zaman, K.; Lawrence, R.; Barnes, J.L.; Fernandes, G. Food Restriction and Fish Oil Suppress Atherogenic Risk Factors in Lupus-Prone (NZB × NZW) F1 Mice. J. Clin. Immunol. 2002, 23, 23–33. [Google Scholar] [CrossRef]
- Borges, M.C.; dos Santos, F.D.M.M.; Telles, R.W.; Lanna, C.C.D.; Correia, M.I.T. Nutritional status and food intake in patients with systemic lupus erythematosus. Nutrition 2012, 28, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Alarcón-de-la-Lastra, C. An update on diet and nutritional factors in systemic lupus erythematosus management. Nutr. Res. Rev. 2017, 30, 118–137. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Sagar, D.; Gaddipati, R.; Ongstad, E.L.; Bhagroo, N.; An, L.L.; Wang, J.; Belkhodja, M.; Rahman, S.; Manna, Z.; Davis, M.A. LOX-1: A potential driver of cardiovascular risk in SLE patients. PLoS ONE 2020, 15, 1–23. [Google Scholar] [CrossRef]
- Zeller, C.; Appenzeller, S. Cardiovascular Disease in Systemic Lupus Erythematosus: The Role of Traditional and Lupus Related Risk Factors. Cur. Cardiol. Rev. 2008, 4, 116–122. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Kong, M.; Li, X.; Wei, D.; Zhu, X.; Hong, Z.; Ni, M.; Wang, Y.; Dong, A. Systemic Lupus Erythematosus and Cardiovascu-lar Disease: A Mendelian Randomization Study. Front. Immunol. 2022, 13, 908831. [Google Scholar] [CrossRef]
- Kahlenberg, J.M.; Kaplan, M.J. The interplay of inflammation and cardiovascular disease in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 203. [Google Scholar] [CrossRef] [Green Version]
- Mercurio, V.; Lobasso, A.; Barbieri, L.; Parrella, P.; Ciervo, D.; Liccardo, B.; Bonaduce, D.; Tocchetti, C.G.; De Paulis, A.; Rossi, F.W. Inflammatory, serological and vascular determinants of cardiovascular disease in systemic lupus erythematosus patients. Int. J. Mol. Sci. 2019, 20, 2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizk, A.; Gheita, T.A.; Nassef, S.; Abdallah, A. The impact of obesity in systemic lupus erythematosus on disease parameters, quality of life, functional capacity and the risk of atherosclerosis: Obesity in SLE. Int. J. Rheum. Dis. 2012, 15, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Salomão, R.G.; de Carvalho, L.M.; Izumi, C.; Czernisz, É.S.; Rosa, J.C.; Antonini, S.R.R.; Bueno, A.C.; Almada, M.O.R.D.V.; Coelho-Landell, C.D.A.; Jordão, A.A.; et al. Homocysteine, folate, hs-C-reactive protein, tumor necrosis factor alpha and inflammatory proteins: Are these biomarkers related to nutritional status and cardiovascular risk in childhood-onset systemic lupus erythematosus? Pediatr. Rheumatol. Online J. 2018, 16, 4. [Google Scholar] [CrossRef] [Green Version]
- Urowitz, M.B.; Gladman, D.; Ibañez, D.; Fortin, P.; Sanchez-Guerrero, J.; Bae, S.; Clarke, A.; Bernatsky, S.; Gordon, C.; Hanly, J. Accumulation of coronary artery disease risk factors over three years: Data from an international inception cohort. Arthritis Rheum. 2008, 59, 176–180. [Google Scholar] [CrossRef]
- Ryu, H.; Chung, Y. Dyslipidemia promotes germinal center reactions via IL-27. BMB Rep. 2018, 51, 371–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, R.; Dwivedi, M.; Mansuri, M.S. Association of Neuropeptide-Y (NPY) and Phenotype Correlation and Plasma Lipids with Type-II Diabetes. PLoS ONE 2016, 11, e0164437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, C.P.; Avalos, I.; Oeser, A.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Stein, C.M. High prevalence of the metabolic syndrome in patients with systemic lupus erythematosus: Association with disease characteristics and cardiovascular risk factors. Ann. Rheum. Dis. 2007, 66, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Giannelou, M.; Mavragani, C.P. Cardiovascular disease in systemic lupus erythematosus: A comprehensive update. J. Autoimmun. 2017, 82, 1–12. [Google Scholar] [CrossRef]
- Bateman, B.T.; Shaw, K.M.; Kuklina, E.V.; Callaghan, W.M.; Seely, E.W.; Hernández-Díaz, S. Hypertension in Women of Reproductive Age in the United States: NHANES 1999-2008. PLoS ONE 2012, 7, e36171. [Google Scholar] [CrossRef]
- Munguia-Realpozo, P.; Mendoza-Pinto, C.; Sierra Benito, C.; Escarcega, R.O.; Garcia-Carrasco, M.; Mendez Martinez, S.; Etchegaray Morales, I.; Galvez Romero, J.L.; Ruiz-Arguelles, A.; Cervera, R. Systemic lupus erythematosus and hypertension. Autoimmun. Rev. 2019, 18, 102371. [Google Scholar] [CrossRef]
- Ciołkiewicz, M.; Kuryliszyn-Moskal, A.; Klimiuk, P.A. Analysis of correlations between selected endothelial cell activation markers, disease activity, and nailfold capillaroscopy microvascular changes in systemic lupus erythematosus patients. Clin. Rheumatol. 2010, 29, 175–180. [Google Scholar] [CrossRef]
- Bell, J.A.; Hamer, M.; David Batty, G.; Singh-Manoux, A.; Sabia, S.; Kivimaki, M. Combined effect of physical activity and leisure time sitting on long-term risk of incident obesity and metabolic risk factor clustering. Diabetologia 2014, 57, 2048–2056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddigan, J.I.; Ardern, C.I.; Riddell, M.C.; Kuk, J.L. Relation of physical activity to cardiovascular disease mortality and the influence of cardiometabolic risk factors. Am. J. Cardiol. 2011, 108, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Kohl, H.W.; Craig, C.L.; Lambert, E.V.; Inoue, S.; Alkandari, J.R.; Leetongin, G.; Kahlmeier, S.; Andersen, L.B.; Bauman, A.E.; Blair, S.N. The pandemic of physical inactivity: Global action for public health. Lancet 2012, 380, 294–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Dwyer, T.; Durcan, L.; Wilson, F. Exercise and physical activity in systemic lupus erythematosus: A systematic review with meta-analyses. Semin. Arthritis Rheum. 2017, 47, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Margiotta, D.P.E.; Basta, F.; Dolcini, G.; Batani, V.; Vullo, M.L.; Vernuccio, A.; Navarini, L.; Afeltra, A. Physical activity and sedentary behavior in patients with Systemic Lupus Erythematosus. PLoS ONE 2018, 13, e0193728. [Google Scholar] [CrossRef]
- Mok, C.C. Metabolic syndrome and systemic lupus erythematosus: The connection. Expert. Rev. Clin. Immunol. 2019, 15, 765–775. [Google Scholar] [CrossRef]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nut. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giannelou, M.; Nezos, A.; Fragkioudaki, S.; Kasara, D.; Maselou, K.; Drakoulis, N.; Ioakeimidis, D.; Moutsopoulos, H.M.; Mavragani, C.P. Contribution of MTHFR gene variants in lupus related subclinical atherosclerosis. Clin. Immunol. 2018, 193, 110–117. [Google Scholar] [CrossRef]
- Sam, N.B.; Zhang, Q.; Li, B.Z.; Li, X.M.; Wang, D.G.; Pan, H.F.; Ye, D.Q. Serum/plasma homocysteine levels in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Clin. Rheumatol. 2020, 39, 1725–1736. [Google Scholar] [CrossRef]
- Timlin, H.; Manno, R.; Douglas, H. Hyperhomocysteinemia and Lupus Nephritis. Cureus 2019, 11, e5065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostopoulou, M.; Nikolopoulos, D.; Parodis, I.; Bertsias, G. Cardiovascular Disease in Systemic Lupus Erythematosus: Recent data on epidemiology, risk factors and prevention. Curr. Vasc. Pharmacol. 2020, 18, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, K.; Sciascia, S.; De Groot, P.G.; Devreese, K.; Jacobsen, S.; Ruiz-Irastroza, G.; Salmon, J.E.; Shoenfeld, Y.; Shovman, O.; Hunt, B.J. Antiphospholipid syndrome. Nat. Rev. Dis. Primers. 2018, 4, 17103. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Laskari, K.; Panagiotakos, D.B.; Moutsopoulos, H.M. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009, 61, 29–36. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Zhou, H.; Xu, Y.; Liu, J.; Xie, H.; Yan, J. Involvement of TLR4 in oxidized LDL/β2GPI/Anti-β2GPI-induced transformation of macrophages to foam cells. J. Atheroscler. Thromb. 2014, 21, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Rho, Y.H.; Chung, C.P.; Oeser, A.; Solus, J.; Raggi, P.; Gebretsadik, T.; Shintani, A.; Stein, C.M. Novel cardiovascular risk factors in premature coronary atherosclerosis associated with systemic lupus erythematosus. J. Rheumatol. 2008, 35, 1789–1794. [Google Scholar]
- Atisha-Fregoso, Y.; Lima, G.; Carrillo-Maravilla, E.; Posadas-Sánchez, R.; Pérez-Hernández, N.; Baños-Peláez, M.; Iturralde-Chávez, A.; Hernández-Díaz, N.; Jakez-Ocampo, J.; Rodríguez-Pérez, J.M. C-reactive protein (CRP) polymorphisms and haplotypes are associated with SLE susceptibility and activity but not with serum CRP levels in Mexican population. Clin. Rheumatol. 2018, 37, 1817–1824. [Google Scholar] [CrossRef]
- Momiyama, Y.; Ohmori, R.; Fayad, Z.A.; Kihara, T.; Tanaka, N.; Kato, R.; Taniguchi, H.; Nagata, M.; Nakamura, H.; Ohsuzu, F. Associations between plasma C-reactive protein levels and the severities of coronary and aortic atherosclerosis. J. Atheroscler. Thromb. 2010, 17, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.M.; Li, C.I.; Kardia, S.L.; Liu, C.S.; Lin, W.Y.; Lee, Y.D.; Chang, P.C.; Lin, C.C.; Li, T.C. Sex difference in the association of metabolic syndrome with high sensitivity C-reactive protein in a Taiwanese population. BMC Public Health 2010, 10, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wee, C.C.; Mukamal, K.J.; Huang, A.; Davis, R.B.; McCarthy, E.P.; Mittleman, M.A. Obesity and C-reactive protein levels among white, black, and hispanic US adults. Obesity 2008, 16, 875–880. [Google Scholar] [CrossRef]
- Flores-Alfaro, E.; Fernández-Tilapa, G.; Salazar-Martínez, E.; Cruz, M.; Illades-Aguiar, B.; Parra-Rojas, I. Common variants in the CRP gene are associated with serum C-reactive protein levels and body mass index in healthy individuals in Mexico. Genet. Mol. Res. 2012, 11, 2258–2267. [Google Scholar] [CrossRef]
- Hanly, J.G.; O’Keeffe, A.G.; Su, L.; Urowitz, M.B.; Romero-Diaz, J.; Gordon, C.; Bae, S.C.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J. The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatology 2015, 55, 252–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermansen, M.L.F.; Lindhardsen, J.; Torp-Pedersen, C.; Faurschou, M.; Jacobsen, S. Incidence of systemic lupus erythematosus and lupus nephritis in Denmark: A nationwide cohort study. J. Rheumatol. 2016, 43, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Maningding, E.; Dall’Era, M.; Trupin, L.; Murphy, L.B.; Yazdany, J. Racial and Ethnic Differences in the Prevalence and Time to Onset of Manifestations of Systemic Lupus Erythematosus: The California Lupus Surveillance Project. Arthritis Care Res. 2020, 72, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.K.; Ward, M.M. Nephritis and the risk of acute myocardial infarction in patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2010, 28, 223–229. [Google Scholar]
- Formiga, F.; Meco, J.F.; Pinto, X.; Jacob, J.; Moga, I.; Pujol, R. Lipid and lipoprotein levels in premenopausal systemic lupus erythematosus patients. Lupus 2001, 10, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, S.; Messedi, M.; Akrout, R.; Ben Hamad, M.; Turki, M.; Marzouk, S.; Keskes, L.; Bahloul, Z.; Rebai, A.; Ayedi, F.; et al. Association of hyperhomocysteinemia with genetic variants in key enzymes of homocysteine metabolism and methotrexate toxicity in rheumatoid arthritis patients. Inflamm. Res. 2018, 67, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Oosterom, N.; de Jonge, R.; Smith, D.E.C.; Pieters, R.; Tissing, W.J.E.; Fiocco, M.; van Zelst, B.D.; van den Heuvel-Eibrink, M.M.; Heil, S.G. Changes in intracellular folate metabolism during high-dose methotrexate and Leucovorin rescue therapy in children with acute lymphoblastic leukemia. Tiziani S, editor. PLoS ONE 2019, 14, e0221591. [Google Scholar] [CrossRef] [Green Version]
- Costedoat-Chalumeau, N.; Dunogué, B.; Morel, N.; Le Guern, V.; Guettrot-Imbert, G. Hydroxychloroquine: A multifaceted treatment in lupus. Presse. Med. 2014, 43, e167–e180. [Google Scholar] [CrossRef]
- Tao, C.Y.; Shang, J.; Chen, T.; Yu, D.; Jiang, Y.M.; Liu, D.; Cheng, G.Y.; Xiao, J.; Zhao, Z.Z. Impact of antimalarial (AM) on serum lipids in systemic lupus erythematosus (SLE) patients: A systematic review and meta-analysis. Medicine 2019, 98, e15030. [Google Scholar] [CrossRef]
- Floris, A.; Piga, M.; Mangoni, A.A.; Bortoluzzi, A.; Erre, G.L.; Cauli, A. Protective Effects of Hydroxychloroquine against Accelerated Atherosclerosis in Systemic Lupus Erythematosus. Mediat. Inflamm. 2018, 2018, 3424136. [Google Scholar] [CrossRef] [Green Version]
- Pagler, T.A.; Neuhofer, A.; Laggner, H.; Strobl, W.; Stangl, H. Cholesterol efflux via HDL resecretion occurs when cholesterol transport out of the lysosome is impaired. J. Lipid Res. 2007, 48, 2141–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ignatescu, M.C.; Kletzmayr, J.; Födinger, M.; Bieglmayer, C.; Hörl, W.H.; Sunder-Plassmann, G. Influence of mycophenolic acid and tacrolimus on homocysteine metabolism. Kidney Int. 2002, 61, 1894–1898. [Google Scholar] [CrossRef] [Green Version]
- Segal, R.; Baumoehl, Y.; Elkayam, O.; Levartovsky, D.; Litinsky, I.; Paran, D.; Wigler, I.; Habot, B.; Leibovitz, A.; Sela, B.A.; et al. anemia, serum vitamin B12, and folic acid in patients with rheumatoid arthritis, psoriatic arthritis, and systemic lupus erythematosus. Rheumatol. Int. 2004, 24, 14–19. [Google Scholar] [CrossRef]
- Hunter, P.M.; Hegele, R.A. Functional foods and dietary supplements for the management of dyslipidaemia. Nat. Rev. En-docrinol. 2017, 13, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Babiker, R.; Elmusharaf, K.; Keogh, M.B.; Saeed, A.M. Effect of Gum Arabic (Acacia Senegal) supplementation on visceral adiposity index (VAI) and blood pressure in patients with type 2 diabetes mellitus as indicators of cardiovascular disease (CVD): A randomized and placebo-controlled clinical trial. Lipids Health Dis. 2018, 17, 56. [Google Scholar] [CrossRef] [PubMed]
- Minami, Y.; Sasaki, T.; Arai, Y.; Kurisu, Y.; Hisamichi, S. Diet and Systemic Lupus Erythematosus: A 4 Year Prospective Study of Japanese Patients. J. Rheumatol. 2003, 30, 747–754. [Google Scholar]
- Selhub, J.; Morris, M.S.; Jacques, P.F. In vitamin B 12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc. Natl. Acad. Sci. USA 2007, 104, 19995–20000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekar, B.; Troyer, D.A.; Venkatraman, J.T.; Fernandes, G. Dietary Omega-3 Lipids Delay the Onset and Progression of Autoimmune Lupus Nephritis by Inhibiting Transforming Growth Factor mRNA and Protein Expression. J. Autoimmun. 1994, 13, 381–393. [Google Scholar] [CrossRef]
- Kinoshita, K.; Yoo, B.S.; Nozaki, Y.; Sugiyama, M.; Ikoma, S.; Ohno, M.; Funauchi, M.; Kanamaru, A. Retinoic Acid Reduces Autoimmune Renal Injury and Increases Survival in NZB/W F 1 Mice. J. Immunol. 2003, 170, 5793–5798. [Google Scholar] [CrossRef] [Green Version]
- Weimann, J.; Weiser, H. Effects of Antioxidant Vitamins C, E, and p-Carotene on Immune Functions in MRL/lpr Mice and Rats. Ann. N. Y. Acad. Sci. 1992, 669, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Strickland, F.M.; Hewagama, A.; Wu, A.; Sawalha, A.H.; Delaney, C.; Hoeltzel, M.F.; Yung, R.; Johnson, K.; Mickelson, B.; Richardson, B.C. Diet Influences Expression of Autoimmune-Associated Genes and Disease Severity by Epigenetic Mechanisms in a Transgenic Mouse Model of Lupus: Diet, DNA Methylation, and Lupus. Arthritis Rheum. 2013, 65, 1872–1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piantoni, S.; Andreoli, L.; Scarsi, M.; Zanola, A.; Dall’Ara, F.; Pizzorni, C.; Cutolo, M.; Airò, P.; Tincani, A. Phenotype modifications of T-cells and their shift toward a Th2 response in patients with systemic lupus erythematosus supplemented with different monthly regimens of vitamin D. Lupus 2015, 24, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Soni, C.; Sinha, I.; Fasnacht, M.J.; Olsen, N.J.; Rahman, Z.S.M.; Sinha, R. Selenium supplementation suppresses immunological and serological features of lupus in B6. Sle1b mice. Autoimmunity 2019, 52, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Lupus erythematosus and nutrition: A review of the literature. J. Ren. Nutr. 2000, 10, 170–183. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Lai, Z.W.; Oaks, Z.; Kelly, R.; Borsuk, R.; Asara, J.M.; Phillips, P.E. Comprehensive metabolome analyses reveal N-acetylcysteine-responsive accumulation of kynurenine in systemic lupus erythematosus: Implications for activation of the mechanistic target of rapamycin. Metabolomics 2015, 11, 1157–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, Z.W.; Hanczko, R.; Bonilla, E.; Caza, T.N.; Clair, B.; Bartos, A.; Miklossy, G.; Jimah, J.; Doherty, E.; Tily, H.; et al. N -acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012, 64, 2937–2946. [Google Scholar] [CrossRef] [Green Version]
- Procaccini, C.; De Rosa, V.; Galgani, M.; Carbone, F.; Cassano, S.; Greco, D.; Qian, K.; Auvinen, P.; Calì, G.; Stallone, G.; et al. Leptin-Induced mTOR Activation Defines a Specific Molecular and Transcriptional Signature Controlling CD4 + Effector T Cell Responses. J. Immunol. 2012, 189, 2941–2953. [Google Scholar] [CrossRef] [Green Version]
- Leiba, A.; Amital, H.; Gershwin, M.E.; Shoenfeld, Y. Diet and lupus. Lupus 2001, 10, 246–248. [Google Scholar] [CrossRef]
- McMahon, M.; Hahn, B.H.; Skaggs, B.J. Systemic lupus erythematosus and cardiovascular disease: Prediction and potential for therapeutic intervention. Expert Rev. Clin. Immunol. 2011, 7, 227–241. [Google Scholar] [CrossRef] [Green Version]
- Pestka, J.J. n-3 Polyunsaturated fatty acids and autoimmune-mediated glomerulonephritis. Prostaglandins Leukot. Essent. Fatty Acids. 2010, 82, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, B.J.; Mann, N.J.; Lewis, J.L.; Milligan, G.C.; Sinclair, A.J.; Howe, P.R.C. Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 2003, 38, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Duffy, E.M.; Meenagh, G.K.; McMILLAN, S.A.; Strain, J.J.; Hannigan, B.M.; Bell, A.L. The Clinical Effect of Dietary Supplementation with Omega-3 Fish Oils and/or Copper in Systemic Lupus Erythematosus. J. Rheumatol. 2004, 31, 1551–1556. [Google Scholar] [PubMed]
- Petri, M. Diet and systemic lupus erythematosus: From mouse and monkey to woman? Lupus 2001, 10, 775–777. [Google Scholar] [CrossRef]
- Fassett, R.G.; Gobe, G.C.; Peake, J.M.; Coombes, J.S. Omega-3 Polyunsaturated Fatty Acids in the Treatment of Kidney Disease. Am. J. Kidney Dis. 2010, 56, 728–742. [Google Scholar] [CrossRef]
- Halade, G.V.; Rahman, M.M.; Bhattacharya, A.; Barnes, J.L.; Chandrasekar, B.; Fernandes, G. Docosahexaenoic Acid-Enriched Fish Oil Attenuates Kidney Disease and Prolongs Median and Maximal Life Span of Autoimmune Lupus-Prone Mice. J. Immunol. 2010, 184, 5280–5286. [Google Scholar] [CrossRef] [Green Version]
- Carracedo, M.; Artiach, G.; Arnardottir, H.; Bäck, M. The resolution of inflammation through omega-3 fatty acids in atherosclerosis, intimal hyperplasia, and vascular calcification. Semin. Immunopathol. 2019, 41, 757–766. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.S.; Desai, T.A.; Wu, B.; Schaller, M.; Werlin, E. Pro-resolving lipid mediators in vascular disease. J. Clin. Invest. 2018, 128, 3727–3735. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Ren, J.; Wei, C.H.; Ross, A.C.; Cecere, T.E.; Jortner, B.S.; Ahmed, S.A.; Luo, X.M. Paradoxical Effects of All-Trans-Retinoic Acid on Lupus-Like Disease in the MRL/lpr Mouse Model. PLoS ONE 2015, 10, e0118176. [Google Scholar] [CrossRef] [Green Version]
- De Lema, G.P.; Lucio-Cazaña, F.J.; Molina, A.N.A.; Luckow, B.; Schmid, H.; de Wit, C.; Moreno-Manzano, V.; Banas, B.; Mampaso, F.; Schlöndorff, D. Retinoic acid treatment protects MRL/lpr lupus mice from the development of glomerular disease. Kidney Int. 2004, 66, 1018–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patavino, T.; Brady, D.M. Natural Medicine and Nutritional Therapy as an Alternative Treatment in Systemic Lupus Erythematosus. Altern. Med. Rev. 2001, 6, 460–471. [Google Scholar] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, E.; Liang, X.M.; Goda, M.; Otani, H.; Mune, M. The efficacy of vitamin E against oxidative damage and autoantibody production in systemic lupus erythematosus: A preliminary study. Clin. Rheumatol. 2007, 26, 401–404. [Google Scholar] [CrossRef]
- Varghese, B.; Haase, N.; Low, P.S. Depletion of Folate-Receptor-Positive Macrophages Leads to Alleviation of Symptoms and Prolonged Survival in Two Murine Models of Systemic Lupus Erythematosus. Mol. Pharm. 2007, 4, 679–685. [Google Scholar] [CrossRef]
- Ardoin, S.; Sandborg, C.; Schanberg, L. Review: Management of dyslipidemia in children and adolescents with systemic lupus erythematosus. Lupus 2007, 16, 618–626. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Dankers, W.; Colin, E.M.; van Hamburg, J.P.; Lubberts, E. Vitamin D in Autoimmunity: Molecular Mechanisms and Therapeutic Potential. Front. Immunol. 2017, 7, 697. [Google Scholar] [CrossRef] [Green Version]
- Illescas-Montes, R.; Melguizo-Rodríguez, L.; Ruiz, C.; Costela-Ruiz, V.J. Vitamin D and autoimmune diseases. Life Sci. 2019, 233, 116744. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Adorini, L. 1α,25-Dihydroxyvitamin D 3 Inhibits Differentiation, Maturation, Activation, and Survival of Dendritic Cells Leading to Impaired Alloreactive T Cell Activation. J Immunol 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolders, J.; Peelen, E.; Thewissen, M.; Cohen Tervaert, J.W.; Menheere, P.; Hupperts, R.; Damoiseaux, J. Safety and T Cell Modulating Effects of High Dose Vitamin D3 Supplementation in Multiple Sclerosis. PLoS ONE 2010, 5, e15235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavi Arab, F.; Rastin, M.; Faraji, F.; Zamani Taghizadeh Rabe, S.; Tabasi, N.; Khazaee, M.; Haghmorad, D.; Mahmoudi, M. Assessment of 1,25-dihydroxyvitamin D3 effects on Treg cells in a mouse model of systemic lupus erythematosus. Immunopharmacol. Immunotoxicol. 2015, 37, 12–18. [Google Scholar] [CrossRef]
- Lemire, J.M.; Ince, A.; Takashima, M. 1,25-Dihydroxyvitamin D 3 Attenuates of Expression of Experimental Murine Lupus of MRL/1 Mice. Autoimmunity 1992, 12, 143–148. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Haque, S.; Williamson, K.; Ray, D.W.; Alexander, M.Y.; Bruce, I.N. Vitamin D improves endothelial dysfunction and restores myeloid angiogenic cell function via reduced CXCL-10 expression in systemic lupus erythematosus. Sci. Rep. 2016, 6, 22341. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Bassuk, S.S.; Lee, I.M.; Cook, N.R.; Albert, M.A.; Gordon, D.; Zaharris, E.; MacFadyen, J.G.; Danielson, E.; Lin, J.; et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp. Clin. Trials. 2012, 33, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Jiao, H.; Acar, G.; Robinson, G.A.; Ciurtin, C.; Jury, E.C.; Kalea, A.Z. Diet and Systemic Lupus Erythematosus (SLE): From Supplementation to Intervention. IJERPH 2022, 19, 11895. [Google Scholar] [CrossRef]
- Albert, C.M. Effect of Folic Acid and B-Vitamins on Risk of Cardiovascular Events and Total Mortality among Women at High Risk for Cardiovascular Disease: A Randomized Trial. JAMA 2009, 299, 2027–2036. [Google Scholar] [CrossRef] [Green Version]
- Lozovoy, M.A.B.; Simão, A.N.C.; Morimoto, H.K.; Scavuzzi, B.M.; Iriyoda, T.V.M.; Reiche, E.M.V.; Cecchini, R.; Dichi, I. Fish oil n-3 fatty acids increase adiponectin and decrease leptin levels in patients with systemic lupus erythematosus. Marine Drugs. 2015, 13, 1071–1083. [Google Scholar] [CrossRef] [Green Version]
- Wright, S.; O’Prey, F.M.; McHenry, M.T.; Leahey, W.J.; Devine, A.B.; Duffy, E.M.; Johnston, D.G.; Finch, M.B.; Bell, A.L.; McVeigh, G.E. A randomised interventional trial of ω-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2008, 67, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, D.; Szponar, B.; Paściak, M.; Czajkowska, A.; Szmyrka, M. Serum levels of n-3 and n-6 polyunsaturated fatty acids in patients with systemic lupus erythematosus and their association with disease activity: A pilot study. Scand. J. Rheumatol. 2022, 51, 230–236. [Google Scholar] [CrossRef]
- Tam, L.S.; Li, E.K.; Leung, V.Y.F.; Griffith, J.F.; Benzie, I.F.F.; Lim, P.L.; Whitney, B.; Lee, V.W.Y.; Lee, K.K.C.; Thomas, G.N.; et al. Effects of vitamins C and E on oxidative stress markers and endothelial function in patients with systemic lupus erythematosus: A double blind, placebo controlled pilot study. J. Rheumatol. 2005, 32, 275–282. [Google Scholar] [PubMed]
- Vianna, A.C.A.; Mocelin, A.J.; Matsuo, T.; Morais-Filho, D.; Largura, A.; Delfino, V.A.; Soares, A.E.; Matni, A.M. Uremic hyperhomocysteinemia: A randomized trial of folate treatment for the prevention of cardiovascular events. Hemodial. Int. 2007, 11, 210–216. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, C.; Guo, H.; Wang, J.; Lin, S.; Li, H.; Yang, Y.; Ling, W. Treatment of coenzyme Q10 for 24 weeks improves lipid and glycemic profile in dyslipidemic individuals. J. Clin. Lipidol. 2018, 12, 417–427.e5. [Google Scholar] [CrossRef]
- Kießling, G.; Schneider, J.; Jahreis, G. Long-term consumption of fermented dairy products over 6 months increases HDL cholesterol. Eur. J. Clin. Nutr. 2002, 56, 843–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, S.; Misra, A.; Pandey, R.M. Effects of 3 g of soluble fiber from oats on lipid levels of Asian Indians—A randomized controlled, parallel arm study. Lipids Health Dis. 2017, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Mottaghi, A.; Ebrahimof, S.; Angoorani, P.; Saboor-Yaraghi, A.A. Vitamin A Supplementation Reduces IL-17 and RORc Gene Expression in Atherosclerotic Patients. Scand. J. Immunol. 2014, 80, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, B.H.; Mohammad Hosseinzadeh, F.; Alipoor, E.; Asghari, S.; Yekaninejad, M.S.; Hosseinzadeh-Attar, M.J. Effects of Selenium Supplementation on Asymmetric Dimethylarginine and Cardiometabolic Risk Factors in Patients with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2020, 196, 430–437. [Google Scholar] [CrossRef]
- Nuttall, S.L. Cardiovascular risk in systemic lupus erythematosus--evidence of increased oxidative stress and dyslipidaemia. Rheumatology 2003, 42, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Wójcik, P.; Gęgotek, A.; Žarković, N.; Skrzydlewska, E. Oxidative Stress and Lipid Mediators Modulate Immune Cell Functions in Autoimmune Diseases. IJMS 2021, 22, 723. [Google Scholar] [CrossRef] [PubMed]
- Hlais, S.; Reslan, D.R.A.; Sarieddine, H.K.; Nasreddine, L.; Taan, G.; Azar, S.; Obeid, O.A. Effect of Lysine, Vitamin B6, and Carnitine Supplementation on the Lipid Profile of Male Patients With Hypertriglyceridemia: A 12-Week, Open-Label, Randomized, Placebo-Controlled Trial. Clin. Ther. 2012, 34, 1674–1682. [Google Scholar] [CrossRef] [PubMed]



| Dietary Component | Model System | Pathophysiological Process | Effects | Reference |
|---|---|---|---|---|
| Polyunsaturated fatty acids (EPA and DHA) | Weanling female (NZB × NZW) F1(B/W) mice | Reduction in IL-1β, TNF-α, and ICAM-1 expression | Delay the onset and progression of lupus nephritis | [72] |
| Vitamin A | NZB/WF mice | Reduction in IFN-γ, IL-2, and anti-DNA levels. Reduction in glomerular deposits of IgG2a | Alleviates autoimmune tissue injuries and prolongs survival | [73] |
| Vitamin C, E and β carotene | MRL/lpr mice | Decrease IgG and anti-dsDNA levels | Possible decrease in SLE symptoms | [74] |
| B vitamins | C57BL/6 mice | Decrease CD40L expression and hematuria | Ameliorate SLE disease | [75] |
| Vitamin D | Premenopausal women with SLE | Increases the number of T-reg cells and reduces the number of CD8+ CD28- T cells | Lowers clinical disease activity | [76] |
| Selenium | NZB/NZW-F1 mice | Inhibits activation, differentiation, and maturation of B cells and macrophages, reduction in autoantibodies to dsDNA | Lowers clinical disease activity | [77] |
| Source | Study Group | Effective Dose and Duration | The Expected Outcome in CVD | Reference |
|---|---|---|---|---|
| n-3 PUFA | Randomized interventional trial in 69 SLE patients from Northern Ireland. | 3 mg for 24 weeks Omacor (omega-3 acid ethyl esters) 4 capsules per day provided 1.8 g EPA and 1.2 g DHA. | Decrease in clinical disease activity by reduction in SLAM-R score from 9.4 to 6.3. Improved endothelial function by increasing flow-mediated dilatation from 3 to 5.7%. | [111] |
| A comparative observational study of 62 SLE patients from Brazil. |
Fish oil 3 g/day for 120 days. | Increased adiponectin levels and decreased leptin levels. | [110] | |
| Vitamin C and E | A double-blind placebo-controlled pilot study in 39 SLE patients from Hong Kong. | Pill with 500 mg of vitamin C and 800 UI vitamin E (D-α tocopherol succinate) for 12 weeks. | Decreased lipid peroxidation measured by a reduction in malondialdehyde concentration. | [113] |
| B vitamins | A randomized, double-blind, placebo-controlled trial in 8171 women with coronary risk factors from the USA | Combination pill containing 2.5 mg of folic acid, 50 mg of B6, and 1 mg of B12 | Decrease in geometric mean homocysteine levels by 18.5%. | [109] |
| A randomized, double-blind, placebo-controlled trial in 186 patients with end-stage kidney disease from Brazil. | Oral folic acid 10 mg, 3 times a week for 2 years. | Decrease in carotid artery intima-media wall thickness from 1.94 ± 0.59 mm to 1.67 ± 0.38 mm. Decrease in homocysteine levels from 25 µmol/L to 10.5 µmol/L. | [114] | |
| Coenzyme Q10 | A randomized, double-blind, placebo-controlled trial in 101 subjects with dyslipidemia. | 30 mg/day of coQ10 for 24 weeks. | Decrease in LDL-C by 6.5%, triglycerides by 19.90%, and serum insulin by 21.09%. | [115] |
| Probiotics | Cross-over study in 29 women with and without hypercholesterolemia from Germany. | 300 g/day of yogurt for 21 weeks. | Increase in HDL-C concentration by 0.3 mmol/L. The ratio of LDL-C/HDL-C decreased from 3.24 to 2.48. | [116] |
| Dietary fiber | A randomized, double-blind, placebo-controlled trial in 91 type 2 diabetic patients. | 30 g/day of soluble fiber (Gum Arabic) for 3 months. | Decrease in systolic blood pressure by 7.6% and decrease in visceral adiposity index by 23.7%. | [69] |
| A randomized, double-blind, placebo-controlled trial in 80 mildly hypercholesterolemic Asian Indians. | 3 g of soluble fiber from 70 g/day of oats. | Decrease in cholesterol levels by 8.1% and decrease in LDL-C levels by 11.6%. | [117] | |
| Vitamin A | A double-blind study in 31 atherosclerotic patients and 15 healthy controls. | 25,000 IU/day of retinyl palmitate for 4 months. | Decrease in IL-17 gene expression by 0.63-fold in fresh cells and 0.82-fold in PHA-activated T cells. | [118] |
| Selenium | A randomized, double-blind, placebo-controlled trial in 66 women with polycystic ovary syndrome. | 200 µg/day of selenium for 12 weeks. | ADMA concentration decreased from 85.14 ± 75 to 56.4 ± 38.6 ng/L. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesqueda-Cendejas, K.; Rivera-Escoto, M.; Meza-Meza, M.R.; Campos-López, B.; Parra-Rojas, I.; Montoya-Buelna, M.; De la Cruz-Mosso, U. Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review. Nutrients 2023, 15, 1036. https://doi.org/10.3390/nu15041036
Pesqueda-Cendejas K, Rivera-Escoto M, Meza-Meza MR, Campos-López B, Parra-Rojas I, Montoya-Buelna M, De la Cruz-Mosso U. Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review. Nutrients. 2023; 15(4):1036. https://doi.org/10.3390/nu15041036
Chicago/Turabian StylePesqueda-Cendejas, Karen, Melissa Rivera-Escoto, Mónica R. Meza-Meza, Bertha Campos-López, Isela Parra-Rojas, Margarita Montoya-Buelna, and Ulises De la Cruz-Mosso. 2023. "Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review" Nutrients 15, no. 4: 1036. https://doi.org/10.3390/nu15041036
APA StylePesqueda-Cendejas, K., Rivera-Escoto, M., Meza-Meza, M. R., Campos-López, B., Parra-Rojas, I., Montoya-Buelna, M., & De la Cruz-Mosso, U. (2023). Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review. Nutrients, 15(4), 1036. https://doi.org/10.3390/nu15041036