Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Analysis of (Poly)phenol Metabolites in Urine Using UHPLC-MS
2.3. Dietary (Poly)phenol Intake Assessment via FFQs
2.4. Gut Microbiome Analysis
2.5. Measurements of Cardiovascular Risk Scores
2.6. Assessment of Covariates
2.7. Statistical Analysis
3. Results
3.1. Population Characteristics
3.2. Urinary (Poly)phenol Metabolites and (Poly)phenol Intake Measured from FFQs
3.3. Urinary (Poly)phenol Metabolites and Gut Microbiome Diversity and Composition
3.4. Circulating (Poly)phenol Metabolites and Cardiovascular Risk Scores
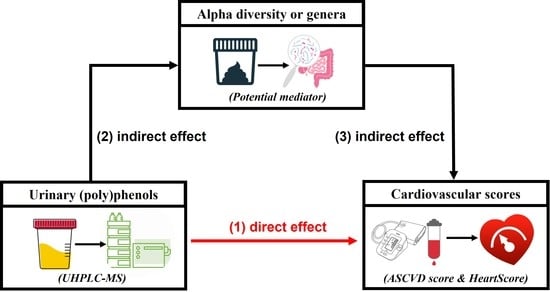
3.5. Urinary Metabolites, Gut Microbiome Composition, and Cardiovascular Risk Score
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perezjimenez, J.; Neveu, V.; Vos, F.; Scalbert, A. Identification of the 100 richest dietary sources of polyphenols: An application of the phenol-explorer database. Eur. J. Clin. Nutr. 2010, 64 (Suppl. 3), S112–S120. [Google Scholar] [CrossRef] [Green Version]
- Mompeo, O.; Spector, T.D.; Hernandez, M.M.; Le Roy, C.; Istas, G.; Le Sayec, M.; Mangino, M.; Jennings, A.; Rodriguez-Mateos, A.; Valdes, A.M.; et al. Consumption of stilbenes and flavonoids is linked to reduced risk of obesity independently of fiber intake. Nutrients 2020, 12, 1871. [Google Scholar] [CrossRef] [PubMed]
- Hemler, E.C.; Hu, F.B. Plant-based diets for cardiovascular disease prevention: All plant foods are not created equal. Curr. Atheroscler. Rep. 2019, 21, 18. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary flavonoid and lignan intake and mortality in prospective cohort studies: Systematic review and dose-response meta-analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Kay, C.; Abdelhamid, A.; Kroon, P.A.; Cohn, J.S.; Rimm, E.B.; Cassidy, A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012, 95, 740–751. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Mateos, A.; Vauzour, D.; Krueger, C.G.; Shanmuganayagam, D.; Reed, J.; Calani, L.; Mena, P.; Del Rio, D.; Crozier, A. Bioavailability, bioactivity and impact on health of dietary flavonoids and related compounds: An update. Arch. Toxicol. 2014, 88, 1803–1853. [Google Scholar] [CrossRef]
- Rechner, A.R.; Smith, M.A.; Kuhnle, G.; Gibson, G.R.; Debnam, E.S.; Srai, S.K.S.; Moore, K.P.; Rice-Evans, C.A. Colonic metabolism of dietary polyphenols: Influence of structure on microbial fermentation products. Free Radic. Biol. Med. 2004, 36, 212–225. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Hervert-Hernández, D.; Sánchez-Mata, M.; Díez-Marqués, C.; Goñi, I. Intestinal bioaccessibility of polyphenols and antioxidant capacity of pulp and seeds of cactus pear. Int. J. Food Sci. Nutr. 2011, 62, 839–843. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The potential influence of fruit polyphenols on colonic microflora and human gut health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Pérez-Martínez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.J.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, E.; Muñoz-González, I.; Jiménez, E.; Bartolomé, B.; Moreno-Arribas, M.V.; Peláez, C.; del Carmen Martínez-Cuesta, M.; Requena, T. Phylogenetic profile of gut microbiota in healthy adults after moderate intake of red wine. Mol. Nutr. Food Res. 2017, 61, 1600620. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mateos, A.; Rendeiro, C.; Bergillos-Meca, T.; Tabatabaee, S.; George, T.W.; Heiss, C.; Spencer, J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013, 98, 1179–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Mateos, A.; Feliciano, R.P.; Boeres, A.; Weber, T.; dos Santos, C.N.; Ventura, M.R.; Heiss, C. Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study. Mol. Nutr. Food Res. 2016, 60, 2130–2140. [Google Scholar] [CrossRef] [Green Version]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of aronia berry (poly)phenols on vascular function and gut microbiota: A double-blind randomized controlled trial in adult men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.; Spector, T.D. Red wine consumption associated with increased gut microbiota α-diversity in 3 independent cohorts. Gastroenterology 2020, 158, 270–272.e272. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Holmes, E.; Wilson, I.D. Gut microorganisms, mammalian metabolism and personalized health care. Nat. Rev. Genet. 2005, 3, 431–438. [Google Scholar] [CrossRef]
- Domínguez-Fernández, M.; Xu, Y.; Yang, P.Y.T.; Alotaibi, W.; Gibson, R.; Hall, W.L.; Barron, L.; Ludwig, I.A.; Cid, C.; Rodriguez-Mateos, A. Quantitative assessment of dietary (poly)phenol intake: A high-throughput targeted metabolomics method for blood and urine samples. J. Agric. Food Chem. 2020, 69, 537–554. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.A.; Welch, A.A.; McTaggart, A.; Mulligan, A.A.; Runswick, S.A.; Luben, R.; Oakes, S.; Khaw, K.T.; Wareham, N.; Day, N.E. Nutritional methods in the european prospective investigation of cancer in Norfolk. Public Health Nutr. 2001, 4, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Phenol-Explorer Database. Available online: http://phenol-explorer.eu/ (accessed on 15 January 2023).
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Isoflavone Content of Selected Foods. 2015. Available online: https://data.nal.usda.gov/dataset/usda-database-isoflavone-content-selected-foods-release-21-november-2015 (accessed on 15 January 2023).
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Proanthocyanidin Content of Selected Foods. 2015. Available online: https://data.nal.usda.gov/dataset/usda-database-proanthocyanidin-content-selected-foods-release-2-2015 (accessed on 15 January 2023).
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Flavonoid Content of Selected Foods. 2016. Available online: https://data.nal.usda.gov/dataset/usda-database-flavonoid-content-selected-foods-release-32-november-2015 (accessed on 15 January 2023).
- Alonso-Esteban, J.I.; Pinela, J.; Ćirić, A.; Calhelha, R.C.; Soković, M.; Ferreira, I.C.; Barros, L.; Torija-Isasa, E.; Sánchez-Mata, M.D.C. Chemical composition and biological activities of whole and dehulled hemp (Cannabis sativa L.) seeds. Food Chem. 2021, 374, 131754. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Jubete, L.; Wijngaard, H.; Arendt, E.; Gallagher, E. Polyphenol composition and in vitro antioxidant activity of amaranth, quinoa buckwheat and wheat as affected by sprouting and baking. Food Chem. 2010, 119, 770–778. [Google Scholar] [CrossRef]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.D.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Micke, G.A.; Tavares, L.B.B.; Fett, R. Nutrient composition and, identification/quantification of major phenolic compounds in Sarcocornia ambigua (Amaranthaceae) using HPLC–ESI-MS/MS. Food Res. Int. 2014, 55, 404–411. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Zhang, Q.; Shen, L.; Luo, J.; Zhu, R.; Mao, J.; Zhao, M.; Cai, C. Phenolic profile and antioxidant activity of Chinese rice wine fermented with different rice materials and starters. LWT—Food Sci. Technol. 2019, 111, 226–234. [Google Scholar] [CrossRef]
- Carvalho, A.V.; da Silveira, T.F.F.; Mattietto, R.D.A.; de Oliveira, M.D.S.P.; Godoy, H.T. Chemical composition and antioxidant capacity of açaí (Euterpe oleracea) genotypes and commercial pulps. J. Sci. Food Agric. 2016, 97, 1467–1474. [Google Scholar] [CrossRef]
- Cicero, N.; Albergamo, A.; Salvo, A.; Bua, G.D.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; Di Stefano, V.; Di Bella, G.; Dugo, G. Chemical characterization of a variety of cold-pressed gourmet oils available on the Brazilian market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef]
- Gao, Q.-H.; Wu, C.-S.; Yu, J.-G.; Wang, M.; Ma, Y.-J.; Li, C.-L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J. Food Sci. 2012, 77, C1218–C1225. [Google Scholar] [CrossRef]
- Gundogdu, M. Determination of antioxidant capacities and biochemical compounds of Berberis vulgaris L. fruits. Adv. Environ. Biol. 2013, 7, 344–348. [Google Scholar]
- Hassan, M.A.; Xu, T.; Tian, Y.; Zhong, Y.; Ali, F.A.Z.; Yang, X.; Lu, B. Health benefits and phenolic compounds of Moringa oleifera leaves: A comprehensive review. Phytomedicine 2021, 93, 153771. [Google Scholar] [CrossRef] [PubMed]
- Karunasiri, A.N.; Gunawardane, M.; Senanayake, C.M.; Jayathilaka, N.; Seneviratne, K.N. Antioxidant and nutritional properties of domestic and commercial coconut milk preparations. Int. J. Food Sci. 2020, 2020, 3489605. [Google Scholar] [CrossRef] [PubMed]
- Kašpar, M.; Bajer, T.; Bajerová, P.; Česla, P. Comparison of phenolic profile of balsamic vinegars determined using liquid and gas chromatography coupled with mass spectrometry. Molecules 2022, 27, 1356. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Luo, F.; Zhao, X.; Liu, Y.; Hu, G.; Sun, C.; Li, X.; Chen, K. Identification of proanthocyanidins from litchi (Litchi chinensis Sonn.) pulp by LC-ESI-Q-TOF-MS and their antioxidant activity. PLoS ONE 2015, 10, e0120480. [Google Scholar] [CrossRef] [Green Version]
- Miceli, N.; Trovato, A.; Marino, A.; Bellinghieri, V.; Melchini, A.; Dugo, P.; Cacciola, F.; Donato, P.; Mondello, L.; Güvenç, A.; et al. Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey. Food Chem. Toxicol. 2011, 49, 2600–2608. [Google Scholar] [CrossRef]
- Mocan, A.; Cairone, F.; Locatelli, M.; Cacciagrano, F.; Carradori, S.; Vodnar, D.C.; Crisan, G.; Simonetti, G.; Cesa, S. Polyphenols from Lycium barbarum (Goji) fruit european cultivars at different maturation steps: Extraction, HPLC-DAD analyses, and biological evaluation. Antioxidants 2019, 8, 562. [Google Scholar] [CrossRef] [Green Version]
- Muala, W.C.B.; Desobgo, Z.S.C.; Jong, N.E. Optimization of extraction conditions of phenolic compounds from Cymbopogon citratus and evaluation of phenolics and aroma profiles of extract. Heliyon 2021, 7, e06744. [Google Scholar] [CrossRef]
- Prasanthi, P.S.; Naveena, N.; Rao, M.V.; Bhaskarachary, K. Compositional variability of nutrients and phytochemicals in corn after processing. J. Food Sci. Technol. 2017, 54, 1080–1090. [Google Scholar] [CrossRef] [Green Version]
- Rahman, J.; de Camargo, A.C.; Shahidi, F. Phenolic and polyphenolic profiles of chia seeds and their in vitro biological activities. J. Funct. Foods 2017, 35, 622–634. [Google Scholar] [CrossRef]
- Rueda, A.; Samaniego-Sánchez, C.; Olalla, M.; Giménez, R.; Cabrera-Vique, C.; Seiquer, I.; Lara, L. Combination of analytical and chemometric methods as a useful tool for the characterization of extra virgin argan oil and other edible virgin oils. Role of polyphenols and tocopherols. J. AOAC Int. 2016, 99, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Du, B.; Xu, B. A systematic, comparative study on the beneficial health components and antioxidant activities of commercially fermented soy products marketed in China. Food Chem. 2015, 174, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Bell, J.T.; Spector, T.D.; Steves, C.J. A heritability-based comparison of methods used to cluster 16S rRNA gene sequences into operational taxonomic units. PeerJ 2016, 4, e2341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mompeo, O.; Berry, S.E.; Spector, T.D.; Menni, C.; Mangino, M.; Gibson, R. Differential associations between a priori diet quality scores and markers of cardiovascular health in women: Cross-sectional analyses from TwinsUK. Br. J. Nutr. 2020, 126, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Braun, L.T.; Ndumele, C.E.; Smith, S.C.; Sperling, L.S.; Virani, S.S.; Blumenthal, R.S. Use of risk assessment tools to guide decision-making in the primary prevention of atherosclerotic cardiovascular disease: A special report from the american heart association and American college of cardiology. J. Am. Coll. Cardiol. 2018, 73, 3153–3167. [Google Scholar] [CrossRef]
- SCORE2 Working Group and ESC Cardiovascular Risk Collaboration. SCORE2 risk prediction algorithms: New models to estimate 10-year risk of cardiovascular disease in Europe. Eur. Heart J. 2021, 42, 2439–2454. [Google Scholar] [CrossRef]
- SCORE2-OP Working Group and ESC Cardiovascular Risk Collaboration. SCORE2-OP risk prediction algorithms: Estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur. Heart J. 2021, 42, 2455–2467. [Google Scholar] [CrossRef]
- Verdi, S.; Abbasian, G.; Bowyer, R.C.E.; Lachance, G.; Yarand, D.; Christofidou, P.; Mangino, M.; Menni, C.; Bell, J.T.; Falchi, M.; et al. TwinsUK: The UK adult twin registry update. Twin Res. Hum. Genet. 2019, 22, 523–529. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Imai, K.; Keele, L.; Tingley, D.; Yamamoto, T. Advances in social science research using R, chap. In Causal Mediation Analysis Using R; Lecture Notes in Statistics; Vinod, H.D., Ed.; Springer: New York, NY, USA, 2010; pp. 129–154. [Google Scholar]
- Li, J.; Ji, L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity 2005, 95, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Londoño, C.; Cayssials, V.; de Villasante, I.; Crous-Bou, M.; Scalbert, A.; Weiderpass, E.; Agudo, A.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Polyphenol intake and epithelial ovarian cancer risk in the european prospective investigation into cancer and nutrition (EPIC) study. Antioxidants 2021, 10, 1249. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European prospective investigation into cancer and nutrition (EPIC) study. Eur. J. Nutr. 2015, 55, 1359–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziauddeen, N.; Rosi, A.; Del Rio, D.; Amoutzopoulos, B.; Nicholson, S.; Page, P.; Scazzina, F.; Brighenti, F.; Ray, S.; Mena, P. Dietary intake of (poly)phenols in children and adults: Cross-sectional analysis of UK national diet and nutrition survey rolling programme (2008–2014). Eur. J. Nutr. 2018, 58, 3183–3198. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ji, S.; Yan, H.; Hao, Y.; Zhang, J.; Wang, Y.; Cao, Z.; Li, S. The day-to-day stability of the ruminal and fecal microbiota in lactating dairy cows. MicrobiologyOpen 2020, 9, e990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Liu, Y.; Nie, C.; Chen, C.; Niu, J.; Zhang, W. Comparison of bacterial and fungal community structure and potential function analysis of yak feces before and after weaning. Bio. Med. Res. Int. 2022, 2022, 6297231. [Google Scholar] [CrossRef] [PubMed]
- Kameda, M.; Abiko, Y.; Washio, J.; Tanner, A.C.R.; Kressirer, C.A.; Mizoguchi, I.; Takahashi, N. Sugar metabolism of Scardovia wiggsiae, a novel caries-associated bacterium. Front. Microbiol. 2020, 11, 479. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E.; et al. Dietary intakes and food sources of phenolic acids in the European prospective investigation into cancer and nutrition (EPIC) study. Br. J. Nutr. 2013, 110, 1500–1511. [Google Scholar] [CrossRef] [Green Version]
- Lavefve, L.; Howard, L.R.; Carbonero, F. Berry polyphenols metabolism and impact on human gut microbiota and health. Food Funct. 2019, 11, 45–65. [Google Scholar] [CrossRef]
- Afnan; Saleem, A.; Akhtar, M.F.; Sharif, A.; Akhtar, B.; Siddique, R.; Ashraf, G.M.; Alghamdi, B.S.; Alharthy, S.A. Anticancer, cardio-protective and anti-inflammatory potential of natural-sources-derived phenolic acids. Molecules 2022, 27, 7286. [Google Scholar] [CrossRef]
- Alborzi, A.; Attar, A.; Sayadi, M.; Nouri, F. The effects of intensive blood pressure control on cardiovascular outcomes based on 10-year ASCVD risk score: An analysis of a clinical trial. Cardiol. Res. Pract. 2021, 2021, 6635345. [Google Scholar] [CrossRef]
- Selma, M.V.; Espiín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Q.; Chen, D.; Yu, B.; He, J. Benzoic acid used as food and feed additives can regulate gut functions. Bio. Med. Res. Int. 2019, 2019, 5721585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, W.; Zhang, P.; Ying, D.; Fang, Z. Hydroxycinnamic acids on gut microbiota and health. Compr. Rev. Food Sci. Food Saf. 2020, 20, 710–737. [Google Scholar] [CrossRef] [PubMed]
- Le Sayec, M.; Xu, Y.; Laiola, M.; Gallego, F.A.; Katsikioti, D.; Durbidge, C.; Kivisild, U.; Armes, S.; Lecomte, M.; Fança-Berthon, P.; et al. The effects of Aronia berry (poly)phenol supplementation on arterial function and the gut microbiome in middle aged men and women: Results from a randomized controlled trial. Clin. Nutr. 2022, 41, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M.; et al. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxidative Med. Cell Longev. 2021, 2021, 6331630. [Google Scholar] [CrossRef]
- Lampe, J.W. Is equol the key to the efficacy of soy foods? Am. J. Clin. Nutr. 2009, 89, 1664S–1667S. [Google Scholar] [CrossRef] [Green Version]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef]
- Xiong, H.-H.; Lin, S.-Y.; Chen, L.-L.; Ouyang, K.-H.; Wang, W.-J. The interaction between flavonoids and intestinal microbes: A review. Foods 2023, 12, 320. [Google Scholar] [CrossRef]
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive oil effects on colorectal cancer. Nutrients 2018, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The firmicutes/bacteroidetes ratio: A relevant marker of gut dysbiosis in obese patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Canani, R.B.; Di Costanzo, M.; Leone, L.; Pedata, M.; Meli, R.; Calignano, A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J. Gastroenterol. 2011, 17, 1519–1528. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarojini, S. Diet, Microbiome and Health; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.-H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astudillo, A.A.; Mayrovitz, H.N. The gut microbiome and cardiovascular disease. Cureus 2021, 13, 14519. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Le Sayec, M.; Roberts, C.; Hein, S.; Rodriguez-Mateos, A.; Gibson, R. Dietary assessment methods to estimate (poly)phenol intake in epidemiological studies: A systematic review. Adv. Nutr. Int. Rev. J. 2021, 12, 1781–1801. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; El Mohsen, M.M.A.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Br. J. Nutr. 2007, 99, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Li, Y.; Ma, X.; Alotaibi, W.; Le Sayec, M.; Cheok, A.; Wood, E.; Hein, S.; Yang, P.Y.T.; Hall, W.L.; et al. Comparison between dietary assessment methods and biomarkers in estimating dietary (poly)phenol intake. Food Funct. 2023, 14, 1369–1386. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | TwinsUK |
|---|---|
| n (%) | |
| MZ | 45 (45%) |
| DZ | 55 (55%) |
| White | 198 (99%) |
| Mean (SD) | |
| Age, yrs | 62.0 (10.0) |
| BMI, kg/m2 | 26.2 (4.7) |
| Fibre intake, g/d | 19.4 (7.0) |
| Energy intake, kcal | 1782.5 (545.7) |
| Measurements of alpha diversity | |
| Shannon Diversity | 5.2 (0.7) |
| Observed OTUs number | 336.9 (106.2) |
| Cardiovascular risk scores | |
| ASCVD risk score | 8.1 (9.9) |
| HeartScore | 9.3 (7.5) |
| Genera | Cardiovascular Score | stdBeta (95% CI) | FDR-Adjusted p-Value |
|---|---|---|---|
| 5-7N15 | ASCVD risk score | −0.05 (−0.09, −0.01) | 0.02 |
| Scardovia | ASCVD risk score | −0.04 (−0.08, −0.004) | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xu, Y.; Le Roy, C.; Hu, J.; Steves, C.J.; Bell, J.T.; Spector, T.D.; Gibson, R.; Menni, C.; Rodriguez-Mateos, A. Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort. Nutrients 2023, 15, 1900. https://doi.org/10.3390/nu15081900
Li Y, Xu Y, Le Roy C, Hu J, Steves CJ, Bell JT, Spector TD, Gibson R, Menni C, Rodriguez-Mateos A. Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort. Nutrients. 2023; 15(8):1900. https://doi.org/10.3390/nu15081900
Chicago/Turabian StyleLi, Yong, Yifan Xu, Caroline Le Roy, Jiaying Hu, Claire J. Steves, Jordana T. Bell, Tim D. Spector, Rachel Gibson, Cristina Menni, and Ana Rodriguez-Mateos. 2023. "Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort" Nutrients 15, no. 8: 1900. https://doi.org/10.3390/nu15081900
APA StyleLi, Y., Xu, Y., Le Roy, C., Hu, J., Steves, C. J., Bell, J. T., Spector, T. D., Gibson, R., Menni, C., & Rodriguez-Mateos, A. (2023). Interplay between the (Poly)phenol Metabolome, Gut Microbiome, and Cardiovascular Health in Women: A Cross-Sectional Study from the TwinsUK Cohort. Nutrients, 15(8), 1900. https://doi.org/10.3390/nu15081900