Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Fermented Red Ginseng, Red Ginseng and Losartan
2.2. Animal Experiments and Diet
2.3. Estimation of Blood Pressure
2.4. Estimation of Oral Glucose Tolerance Tests
2.5. Estimation of Biochemical Analysis of Plasma
2.6. Protein Preparation and Immunoblotting in the Rat Aorta and Muscle
2.7. Histopathological and Oil Red OStaining of Aortic Tissues, Epididymal Fat and Liver Tissues
2.8. Immunihistochemical Staining of Aortic Tissues
2.9. Statistical Analysis
3. Results
3.1. Effects of FRG on Changes in Body Weight, Liver Weight and Epididymal Fat Pad Weight
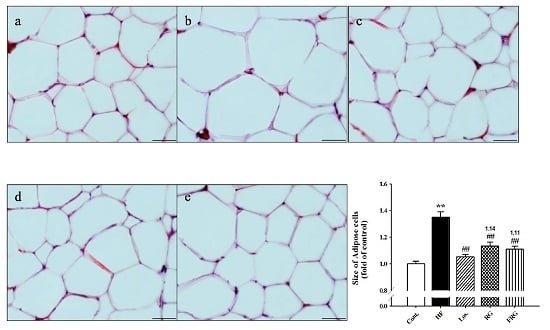
3.2. Effect of FRG on the Morphology of Epididymal Fat Pads
3.3. Effect of FRG on Plasma Lipid Levels
3.4. Effect of FRG on Plasma Parameters
3.5. Effect of FRG on Oral Glucose Tolerance Tests
3.6. Effect of FRG on Blood Pressure
3.7. Effect of FRG on the Morphology of Aortas
3.8. Effect of FRG on the Expressions Levels of Adhesion Molecules and ET-1 in Aortas
3.9. Effect of FRG on Hepatic Lipids
3.10. Effect of FRG on the Expressions Levels of IRS-1 and Glut4in Muscle Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- El-Bassossy, H.M.; Shaltout, H.A. Allopurinol alleviateshypertension and proteinuria in high fructose, high salt and high fat induced model of metabolic syndrome. Transl. Res. 2015, 165, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Journal of the American Medical Association (JAMA). National cholesterol education program: Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (adults treatment panel III). Circulation 2001, 285, 2487–2497. [Google Scholar]
- Tilg, H.; Hotamisligil, G.S. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology 2006, 131, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Shoelson, S.E.; Herrero, L.; Naaz, A. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose or high-fructose corn syrup. Am. J. Clin. Nutr. 2008, 88, 1733S–1737S. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Havel, P.J. Fructose consumption: Recentresults and their potential implications. Ann. N. Y. Acad. Sci. 2010, 1190, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Basciano, H.; Federico, L.; Adeli, K. Fructose, insulin resistance,and metabolic dyslipidemia. Nutr. Metab. 2005, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.Y.; Okubo, T.; Juneja, L.R.; Yokozawa, T. Theprotective role of amla (Emblica officinalis Gaertn.) againstfructose-induced metabolic syndrome in a rat model. Br. J. Nutr. 2010, 103, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumptionas a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Brown, L. Rodent models for metabolicsyndrome research. J. Biomed. Biotechnol. 2011, 2011, 351982. [Google Scholar] [CrossRef] [PubMed]
- Ferder, L.; Ferder, M.D.; Inserra, F. The role of high-fructosecorn syrup in metabolic syndrome and hypertension. Curr. Hypertens. Rep. 2010, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, H.; Yanagita, T.; Yamamoto, K.; Ogawa, Y.; Cha, J.Y.; Mori, Y. Inhibitory effect of Oren-Gedoku-to and its components on cholesterol ester synthesis in cultured human hepatocyte HepG2 cells: Evidence from the cultured HepG2 cells and in vitro assay of ACAT. Planta Med. 1997, 63, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, T.A. Cholesterol absorption inhibition: A strategy for cholesterol-lowering therapy. Int. J. Clin. Pract. 2001, 55, 710–716. [Google Scholar] [PubMed]
- Shin, M.K.; Han, S.H. Effects of methanol extracts from bamboo (Pseudosasa japonica Makino) leaves extracts on lipid metabolism in rats fed high fat and high cholesterol diet. Korean J. Food Cult. 2002, 17, 30–36. [Google Scholar]
- Ko, S.K.; Lee, C.R.; Choi, Y.E.; Im, B.O.; Sung, J.H.; Yoon, K.R. Analysis of ginsenosides of white and red ginseng concentrates. Korean J. Food Sci. Technol. 2003, 35, 536–539. [Google Scholar]
- Kim, N.D. Pharmacological effects of red ginseng. J. Ginseng Res. 2001, 25, 2–10. [Google Scholar]
- Ramesh, T.; Kin, S.W.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr. Res. 2012, 32, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Heo, H.; Oh, S.; Lew, J. Comparison study of Korean and Chinese ginsengs on the regulation of lymphocyte proliferation and cytokine production. J. Ginseng Res. 2008, 32, 250–256. [Google Scholar]
- Lee, H.; Park, D.; Yoon, M. Korean red ginseng (Panax ginseng) prevents obesity by inhibiting angiogenesis in high fat diet induced obes C57BL/6J mice. Food Chem. Toxicol. 2013, 53, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, DH. Metabolism of ginsenosides to bioactive compounds by intestinal microfloraand its industrial application. J. Ginseng Res. 2009, 33, 165–176. [Google Scholar]
- Bae, E.A.; Han, M.J.; Kim, E.J.; Kim, D.H. Transformation of ginseng saponins to ginsenoside Rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch. Pharm. Res. 2004, 27, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Tachibana, S.; Kuba-Miyara, M. Biochemical aspects of red koji and tofuyo preparedusing Monascus fungi. Appl. Microbiol. Biotechnol. 2012, 96, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Trinh, H.T.; Han, S.J.; Kim, S.W.; Lee, Y.C.; Kim, D.H. Bifidus fermentation increaseshypolipidemic and hypoglycemic effects of red ginseng. J. Microbiol. Biotechnol. 2007, 17, 1127–1133. [Google Scholar] [PubMed]
- Kim, H.Y.; Kim, K. Regulation of signaling molecules associated with insulin action, insulin secretion and pancreatic beta-cell mass in the hypoglycemic effect of Korean red ginseng in Goto-Kakizaki rat. J. Ethnopharmacol. 2012, 142, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.S.; Jo, S.H.; Kang, B.H.; Apostolidis, E.; Lee, M.S.; Jang, H.D.; Kwon, Y.I. In vitro and in vivo antihyperglycemic effect of 2 amadori rearrangement compounds, arginyl-fructose and arginyl-fructose-glucose. J. Food Sci. 2011, 76, H188–H193. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Sung, M.K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Buono, M.D.; Lee, K.S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T.; et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [PubMed]
- Kim, B.G.; Choi, S.Y.; Kim, M.R.; Suhd, H.J.; Park, H.J. Changes of ginsenosides in Korean red ginseng (Panax ginseng) fermented by Lactobacillus plantarum M1. Process. Biochem. 2010, 45, 1319–1324. [Google Scholar] [CrossRef]
- Miatello, R.; Vázquez, M.; Renna, N.; Cruzado, M.; Zumino, A.P.; Risler, N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am. J. Hypertens. 2005, 18, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Renna, N.F.; Vazquez, M.A.; Lama, M.C.; González, E.S.; Miatello, R.M. Effect of chronic aspirin administration on an experimental model of metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2009, 36, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L. Matrix metalloproteinases:influence on smooth muscle cells and atherosclerotic plaque stability. Expert. Rev. Cardiovasc. Ther. 2007, 5, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sanders, J.M.; Phan, E.T.; Ley, K.; Sarembock, I.J. Arterial macrophages and regenerating endothelial cells express P-selectin in atherosclerosis-prone apolipoprotein E-deficient mice. Am. J. Pathol. 2005, 167, 1511–1518. [Google Scholar] [CrossRef]
- Endemann, D.H.; Schiffrin, E.L. Nitric oxide, oxidative excess, and vascular complications of diabetes mellitus. Curr. Hypertens. Rep. 2004, 6, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halass, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 1, 1155–1161. [Google Scholar]
- Carter, C.S.; Giovannini, S.; Seo, D.O.; DuPree, J.; Morgan, D.; Chung, H.Y.; Lees, H.; Daniels, M.; Hubbard, G.B.; Lee, S.; et al. Differential effects of enalapril and losartan on body composition and indices of muscle quality in aged male Fischer 344 × Brown Norway rats. Age 2011, 33, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Wellen, J.; Delafontaine, P. Angiotensin II causes weight loss and decreases circulating insulin-like growth factor I in rats through a pressor-independent mechanism. J. Clin. Invest. 1996, 97, 2509–2516. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Mori, T.; Jiang, Y.; Hu, C.; Ohsaki, Y.; Yoneki, Y.; Nakamichi, T.; Ogawa, S.; Sato, H.; Ito, S. Losartan modulates muscular capillary density and reverses thiazide diuretic-exacerbated insulin resistance in fructose-fed rats. Hypertens. Res. 2012, 35, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Saha, A.K.; Xiang, X.; Ruderman, N.B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005, 26, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Majithiya, J.B.; Balaraman, R. Metformin reduces blood pressure and restores endothelial function in aorta of streptozotocin-induced diabetic rats. Life Sci. 2006, 78, 2615–2624. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Adeli, K. Dietary fructose and the metabolic syndrome. Curr. Opin. Gastroenterol. 2008, 24, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Yuen, V.G.; McNeill, J.H. The fructose-fed rat: A review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol. Cell. Biochem. 2009, 332, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Thong, F.S.L.; Bilan, P.J.; Klip, A. The RabGTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes 2007, 56, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Maianu, L.; Zhu, J.H.; Brechtel-Hook, G.; Wallace, P.; Baron, A.D. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 1998, 101, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.W.; Elloitt, B.T. Akt/PKB activation and insulin signaling: A novel insulin signaling pathway in the treatment of type 2 diabets. Diabetes Metab. Syndr. Obes. 2014, 7, 55–64. [Google Scholar] [CrossRef] [PubMed]








| Groups | Control | HF | HF | ||
|---|---|---|---|---|---|
| Los. | RG | FRG | |||
| Initial BW (g) | 270.5 ± 3.3 | 270.0 ± 3.1 | 275.8 ± 3.6 | 272.6 ± 1.8 | 275.5 ± 4.3 |
| Terminal BW (g) | 399.6 ± 7.4 | 421.2 ± 6.0 | 384.1 ± 9.8 # | 405.8 ± 10.5 | 393.9 ± 6.4 # |
| Food intake (g/day) | 18.5 ± 0.3 | 18.4 ± 0.4 | 18.2 ± 0.5 | 18.8 ± 0.4 | 18.4 ± 0.5 |
| Liver weight (g) | 9.0 ± 1.3 | 12.3 ± 0.4 * | 9.2 ± 0.4 ## | 10.5 ± 0.7 | 9.9 ± 0.3 # |
| Epididymal fat pads weight (g) | 6.4 ± 0.9 | 9.0 ± 0.4 * | 5.5 ± 0.4 ## | 7.6 ± 0.7 # | 6.6 ± 0.7 # |
| Groups | Control | HF | HF | ||
|---|---|---|---|---|---|
| Los. | RG | FRG | |||
| T-Cho (mg/dL) | 69.1 ± 7.2 | 104.2 ± 11.5 * | 72.5 ± 5.5 # | 80.0 ± 4.1 # | 70.3 ± 7.1 # |
| TG (mg/dL) | 155.0 ± 12.8 | 243.6 ± 27.5 * | 117.8 ± 17.1 ## | 180.3 ± 32.6 | 169.0 ± 25.1 |
| HDL-c (mg/dL) | 57.7 ± 5.5 | 45.2 ± 4.1 | 56.4 ± 1.6 # | 56.0 ± 3.1 # | 60.5 ± 4.4 # |
| LDL-c (mg/dL) | 22.2 ± 2.1 | 30.0 ± 2.6* | 20.2 ± 1.8 # | 26.0 ± 1.6 | 22.2 ± 1.7 # |
| Groups | Control | HF | HF | ||
|---|---|---|---|---|---|
| Los. | RG | FRG | |||
| GOT (IU/L) | 137.9 ± 10.5 | 164.3 ± 15.0 | 155.1 ± 9.8 | 161.8 ± 28.6 | 137.00 ± 16.4 |
| GPT (IU/L) | 22.2 ± 3.8 | 29.3 ± 6.7 | 30.3 ± 4.5 | 26.8 ± 1.8 | 28.1 ± 6.5 |
| T-bill (mg/mL) | 0.49 ± 0.04 | 0.48 ± 0.04 | 0.48 ± 0.03 | 0.49 ± 0.02 | 0.38 ± 0.03 |
| Leptin (ng/mL) | 5.76 ± 1.0 | 10.93 ± 2.0 * | 3.80 ± 0.7 ## | 6.32 ± 1.1 | 5.41 ± 1.3 # |
| Insulin (ng/mL) | 2.1 ± 0.5 | 4.1 ± 0.6 * | 2.0 ± 0.5 # | 2.4 ± 0.6 | 2.2 ± 0.3 # |
| Blood glucose (mg/dL) | 129.4 ± 5.6 | 136.9 ± 3.2 | 128.1 ± 4.6 | 129.4 ± 7.1 | 130.3 ± 2.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kho, M.C.; Lee, Y.J.; Park, J.H.; Kim, H.Y.; Yoon, J.J.; Ahn, Y.M.; Tan, R.; Park, M.C.; Cha, J.D.; Choi, K.M.; et al. Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models. Nutrients 2016, 8, 369. https://doi.org/10.3390/nu8060369
Kho MC, Lee YJ, Park JH, Kim HY, Yoon JJ, Ahn YM, Tan R, Park MC, Cha JD, Choi KM, et al. Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models. Nutrients. 2016; 8(6):369. https://doi.org/10.3390/nu8060369
Chicago/Turabian StyleKho, Min Chul, Yun Jung Lee, Ji Hun Park, Hye Yoom Kim, Jung Joo Yoon, You Mee Ahn, Rui Tan, Min Cheol Park, Jeong Dan Cha, Kyung Min Choi, and et al. 2016. "Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models" Nutrients 8, no. 6: 369. https://doi.org/10.3390/nu8060369
APA StyleKho, M. C., Lee, Y. J., Park, J. H., Kim, H. Y., Yoon, J. J., Ahn, Y. M., Tan, R., Park, M. C., Cha, J. D., Choi, K. M., Kang, D. G., & Lee, H. S. (2016). Fermented Red Ginseng Potentiates Improvement of Metabolic Dysfunction in Metabolic Syndrome Rat Models. Nutrients, 8(6), 369. https://doi.org/10.3390/nu8060369