Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe
Abstract
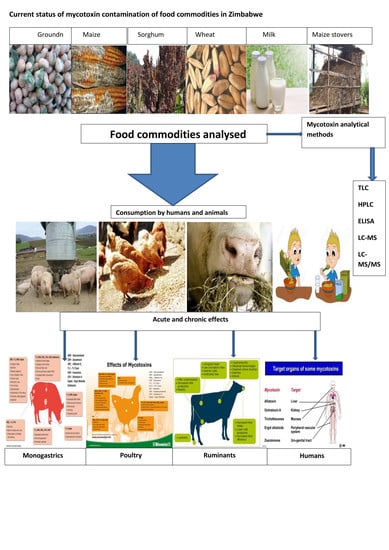
:1. Introduction
2. Methods
3. Occurrence of Toxigenic Fungi in Foods and Feeds in Zimbabwe
4. Aflatoxins
5. Aflatoxins in Food and Feed
6. Fusarium Mycotoxins
7. Mycotoxin Analysis
8. Legislation
9. Discussion
10. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Misihairabgwi, J.; Ezekiel, C.; Sulyok, M.; Shephard, G.; Krska, R. Mycotoxin contamination of foods in Southern Africa: A 10-year review (2007–2016). Crit. Rev. Food Sci. Nutr. 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mupunga, I.; Lebelo, S.L.; Mngqawa, P.; Rheeder, J.; Katerere, D. Natural occurrence of aflatoxins in peanuts and peanut butter from Bulawayo, Zimbabwe. J. Food Prot. 2014, 77, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Datsugwai, M.S.S.; Ezekiel, B.; Audu, Y.; Legbo, M.I.; Azeh, Y.; Gogo, M.R. Mycotoxins: Toxigenic fungal compounds—A review. ARPN J. Sci. Technol. 2013, 3, 687–692. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Guchi, E. Implication of aflatoxin contamination in agricultural products. Am. J. Food Nutr. 2015, 3, 12–20. [Google Scholar]
- Darwish, W.S.; Ikenaka, Y.; Nakayama, S.M.; Ishizuka, M. An overview on mycotoxin contamination of foods in Africa. J. Vet. Med. Sci. 2014, 76, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Mycotoxin problem in Africa: Current status, implications to food safety and health and possible management strategies. Int. J. Food Microbiol. 2008, 124, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2009, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Songsermsakul, P. Mycotoxins contamination of food in Thailand (2000–2010): Food safety concerns for the world food exporter. Int. Food Res. J. 2015, 22, 426–434. [Google Scholar]
- Gallo, A.; Giuberti, G.; Frisvad, J.C.; Bertuzzi, T.; Nielsen, K.F. Review on mycotoxin issues in ruminants: Occurrence in forages, effects of mycotoxin ingestion on health status and animal performance and practical strategies to counteract their negative effects. Toxins 2015, 7, 3057–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsah-Hejri, L.; Jinap, S.; Hajeb, P.; Radu, S.; Shakibazadeh, S. A review on mycotoxins in food and feed: Malaysia case study. Compr. Rev. Food Sci. Food Saf. 2013, 12, 629–651. [Google Scholar] [CrossRef]
- Pessu, P.; Agoda, S.; Isong, I.; Adekalu, O.; Echendu, M.; Falade, T. Fungi and mycotoxins in stored foods. Afr. J. Microbiol. Res. 2011, 5, 4373–4382. [Google Scholar]
- Mansfield, M.; Kuldau, G. Microbiological and molecular determination of mycobiota in fresh and ensiled maize silage. Mycologia 2007, 99, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.M.; El-Midany, S.; Shaheen, H.; Rizzi, L. Mycotoxins in animals: Occurrence, effects, prevention and management. J. Toxicol. Environ. Health Sci. 2012, 4, 13–28. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Murashiki, T.C.; Chidewe, C.; Benhura, M.A.; Maringe, D.T.; Dembedza, M.P.; Manema, L.R.; Mvumi, B.M.; Nyanga, L.K. Levels and daily intake estimates of aflatoxin B1 and fumonisin B1 in maize consumed by rural households in Shamva and Makoni districts of Zimbabwe. Food Control 2017, 72, 105–109. [Google Scholar] [CrossRef]
- Dangwa, N.; Mwenje, E.; Dhlamini, Z.; Siwela, A. Molecular characterization of aflatoxigenic Aspergillus species in dried traditional foods in Zimbabwe. Adv. Biores. 2014, 5, 29–36. [Google Scholar]
- Mubatanhema, W.; Moss, M.O.; Frank, M.J.; Wilson, D.M. Prevalence of Fusarium species of the liseola section on Zimbabwean corn and their ability to produce the mycotoxins zearalenone, moniliformin and fumonisin B1. Mycopathologia 1999, 148, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Gehesquière, S.; De Saeger, P.D.; Hove, M.; Haesaert, G. The Effect of Time on Mycotoxins in Subsistence Farmed Maize from Zimbabwe. Master’s Thesis, Ghent University, Ghent, Belgium, 16 August 2016. [Google Scholar]
- Gamanya, R.; Sibanda, L. Survey of Fusarium moniliforme (F. verticillioides) and production of fumonisin B1 in cereal grains and oilseeds in Zimbabwe. Int. J. Food Microbiol. 2001, 71, 145–149. [Google Scholar] [CrossRef]
- Onyike, N.B.; Nelson, P.E. Fusarium species associated with sorghum grain from Nigeria, Lesotho, and Zimbabwe. Mycologia 1992, 84, 452–458. [Google Scholar] [CrossRef]
- Onyike, N.B.; Nelson, P.E.; Marasas, W. Fusarium species associated with millet grain from Nigeria, Lesotho, and Zimbabwe. Mycologia 1991, 83, 708–712. [Google Scholar] [CrossRef]
- Ibáñez-Vea, M.; González-Peñas, E.; Lizarraga, E.; De Cerain, A.L. Co-occurrence of aflatoxins, ochratoxin a and zearalenone in barley from a northern region of Spain. Food Chem. 2012, 132, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Nleya, N.; Nyararai, Y.; Mawanza, M.; Mlalazi, F. Colonization of stem borer damaged maize plants by aflatoxigenic Aspergillus species in Zimbabwe. Trop. Plant Res. 2017, 4, 109–114. [Google Scholar] [CrossRef]
- Dube, M.; Maphosa, M. Prevalence of aflatoxigenic Aspergillus spp and groundnut resistance in Zimbabwe. IOSR J. Agric. Vet. Sci. 2014, 7, 8–12. [Google Scholar] [CrossRef]
- Maringe, D.T.; Chidewe, C.; Benhura, M.A.; Mvumi, B.M.; Murashiki, T.C.; Dembedza, M.P.; Siziba, L.; Nyanga, L.K. Natural postharvest aflatoxin occurrence in food legumes in the smallholder farming sector of Zimbabwe. Food Add. Contam. Part B 2017, 10, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Siwela, A.H.; Caley, A. Aflatoxin contamination of stored groundnuts in Zimbabwe. In International Proceedings of the International Crops Research Institute Workshop, 6–9 Octomber 1987, ICRISAT for the Semi-Arid Tropics); ICRISAT Center: Patancheru, India, 1989. [Google Scholar]
- Titterton, M. Forage Production and Conservation for Dry Season Feeding of Dairy Cattle in the Semi-Arid Region of Zimbabwe: A Review and Report on Recent Work for a DFID Project; Department for International Development: London, UK, 2001.
- Panigrahi, S. Final Technical Report: The Effects of Storage of Fibrous Feeds on Ruminant Livestock in Developing Countries; Natural Resources Institute (NRI): Warwickshire, UK, 1996; p. 50. [Google Scholar]
- Atherstone, C.; Grace, D.; Lindahl, J.; Kang’ethe, E.; Nelson, F. Assessing the impact of aflatoxin consumption on animal health and productivity. Afr. J. Food Agric. Nutr. Dev. 2016, 16, 10949–10966. [Google Scholar] [CrossRef]
- Baranyi, N.; Kocsubé, S.; Vágvölgyi, C.; Varga, J. Current trends in aflatoxin research. Acta Biol. Szeged. 2013, 57, 95–107. [Google Scholar]
- Arapcheska, M.; Jovanovska, V.; Jankuloski, Z.; Musliu, Z.; Uzunov, R. Impact of aflatoxins on animal and human health. Int. J. Innov. Sci. Eng. Technol. 2015, 2, 156–161. [Google Scholar]
- Reid, C.X.; Sparks, D.L.; Williams, W.P.; Brown, A.E. Single corn kernel aflatoxin b1 extraction and analysis method. Nat. Res. 2016, 7, 405. [Google Scholar]
- Donner, M.; Lichtemberg, P.S.; Doster, M.; Picot, A.; Cotty, P.J.; Puckett, R.D.; Michailides, T.J. Community structure of Aspergillus flavus and A. parasiticus in major almond-producing areas of California, United States. Plant Dis. 2015, 99, 1161–1169. [Google Scholar] [CrossRef]
- Yu, J.; Fedorova, N.D.; Montalbano, B.G.; Bhatnagar, D.; Cleveland, T.E.; Bennett, J.W.; Nierman, W.C. Tight control of mycotoxin biosynthesis gene expression in Aspergillus flavus by temperature as revealed by RNA-seq. FEMS Microbiol. Lett. 2011, 322, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Aflatoxins and aflatoxicosis in human and animals. In Aflatoxins-Biochemistry and Molecular Biology; InTech: Houston, TX, USA, 2011; pp. 221–254. [Google Scholar]
- Abdel-Hadi, A.; Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bbosa, G.S.; Kitya, D.; Odda, J.; Ogwal-Okeng, J. Aflatoxins metabolism, effects on epigenetic mechanisms and their role in carcinogenesis. Health 2013, 5, 14. [Google Scholar] [CrossRef]
- Jawaid, S.; Talpur, F.N.; Nizamani, S.M.; Afridi, H.I. Contamination profile of aflatoxin m1 residues in milk supply chain of Sindh. Toxicol. Rep. 2015, 2, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H. A review of aflatoxin m1, milk, and milk products. In Aflatoxins-Biochemistry and Molecular Biology; InTech: Houston, TX, USA, 2011; pp. 397–414. [Google Scholar]
- Siwela, A.H.; Nziramasanga, N. Regulatory aspects of aflatoxin control in Zimbabwe—A review. J. Appl. Sci. South. Afr. 1999, 5, 141–147. [Google Scholar] [CrossRef]
- Smith, L.E.; Mbuya, M.N.; Prendergast, A.J.; Turner, P.C.; Ruboko, S.; Humphrey, J.H.; Nelson, R.J.; Chigumira, A.; Kembo, G.; Stoltzfus, R.J. Determinants of recent aflatoxin exposure among pregnant women in rural Zimbabwe. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Nyathi, C.B.; Mutiro, C.F.; Hasler, J.A.; Chetsanga, C.J. A survey of urinary aflatoxin in Zimbabwe. Int. J. Epidemiol. 1987, 16, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Pionneau, F.A.; Montesano, R.; Mutiro, C.F.; Chetsanga, C.J. Aflatoxin detected in human breast milk by immunoassay. Int. J. Cancer 1987, 40, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Bandyopadhyay, R.; Cotty, P. Diversity of aflatoxin-producing fungi and their impact on food safety in sub-Saharan Africa. Int. J. Food Microbiol. 2014, 174, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Matsiko, F.; Kanyange, C.; Ingabire, G.; Dusingizimana, T.; Vasanthakaalam, H.; Kimonyo, A. Detection and quantification of aflatoxin in cassava and maize flour sold in Kigali open markets, Rwanda. Int. Food Res. J. 2017, 24, 459–464. [Google Scholar]
- Siwela, A.H. Combined use of phenyl-bonded phase clean-up and hplc for the determination of aflatoxins. Trop. Sci. 1996, 36, 197–200. [Google Scholar]
- Musundire, R.; Osuga, I.M.; Cheseto, X.; Irungu, J.; Torto, B. Aflatoxin contamination detected in nutrient and anti-oxidant rich edible stink bug stored in recycled grain containers. PLoS ONE 2016, 11, e0145914. [Google Scholar] [CrossRef] [PubMed]
- Choga, R.J.S.O.; Chiriseri, B.; Pfukenyi, D.M. Detection and levels of aflatoxin m₁ in raw milk of dairy cows from selected small scale and commercial farms in harare, Zimbabwe. Zimb. Vet. J. 2016, 34, 1–6. [Google Scholar]
- Hove, M.; De Boevre, M.; Lachat, C.; Jacxsens, L.; Nyanga, L.; De Saeger, S. Occurrence and risk assessment of mycotoxins in subsistence farmed maize from Zimbabwe. Food Control 2016, 69, 36–44. [Google Scholar] [CrossRef]
- Morton, J.; Wood, C.; Ncube, S.; Coker, R.; Nyoni, N.; Thomas, D.; Nagler, M. Effects of Post-Harvest Practices on the Production and Nutritive Value of Maize Residues in Zimbabwe; Final Technical Report; Annexes: Herndon, VA, USA, 2000. [Google Scholar]
- Mupunga, I.; Mngqawa, P.; Katerere, D.R. Peanuts, aflatoxins and undernutrition in children in sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Ghiasian, S.; Maghsood, A. Infants’ exposure to aflatoxin M1 from mother’s breast milk in Iran. Iran. J. Public Health 2012, 41, 119. [Google Scholar] [PubMed]
- Siwela, A.H.; Mukaro, K.J.; Nziramasanga, N. Aflatoxin carryover during large scale peanut butter production. Food Nutr. Sci. 2011, 2, 103–108. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Matumba, L.; Kanenga, K.; Siambi, M.; Waliyar, F.; Maruwo, J.; Monyo, E.S. A case for regular aflatoxin monitoring in peanut butter in sub-Saharan Africa: Lessons from a 3-year survey in Zambia. J. Food Prot. 2016, 79, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Ramin, M.; Höjer, A.; Hetta, M. The effects of legume seeds on the lactation performance of dairy cows fed grass silage-based diets. Agric. Food Sci. 2017, 26, 129–137. [Google Scholar] [CrossRef]
- Moog, F. Forage and legumes as protein supplements for pasture based systems. In Feeding Dairy Cows in the Tropics, Proceedings of the FAO Expert Consultation, Bangkok, Thailand, 7–11 July 1989; FAO: Rome, Italy, 1989. [Google Scholar]
- Batterham, E.; Egan, A. Utilization of food legumes as feed. In Food Legume Improvement for Asian Farming Systems; ACIAR: Canberra, Australia, 1986; pp. 193–200. [Google Scholar]
- Oluwafemi, F.; Lawal, S. Hygienic status of cow milk and wara from local Fulani herdsmen in two western states of Nigeria. Br. Microbiol. Res. J. 2015, 5, 389. [Google Scholar] [CrossRef]
- Tajkarimi, M.; Aliabadi-Sh, F.; Nejad, A.S.; Poursoltani, H.; Motallebi, A.; Mahdavi, H. Aflatoxin M1 contamination in winter and summer milk in 14 states in Iran. Food Control 2008, 19, 1033–1036. [Google Scholar] [CrossRef]
- D’mello, J.; Placinta, C.; Macdonald, A. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Technol. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tembo, E. Mycotoxigenic Fungi Associated with Ear-Rots in Zimbabwe: Identification and Inheritance of Resistance in Southern and West African Maize Inbred Lines. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2015. [Google Scholar]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. The status of Fusarium mycotoxins in sub-Saharan Africa: A review of emerging trends and post-harvest mitigation strategies towards food control. Toxins 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Marasas, W. Discovery and occurrence of the fumonisins: A historical perspective. Environ. Health Perspect. 2001, 109, 239. [Google Scholar] [CrossRef] [PubMed]
- Hove, M.; Van Poucke, C.; Njumbe-Ediage, E.; Nyanga, L.; De Saeger, S. Review on the natural co-occurrence of afb1 and fb1 in maize and the combined toxicity of AFB1 and FB1. Food Control 2016, 59, 675–682. [Google Scholar] [CrossRef]
- Mupunga, I. A Comparative Study of Natural Contamination with Aflatoxins and Fumonisins in Selected Food Commodities from Botswana and Zimbabwe. Master’s Thesis, University of South Africa, Pretoria, South Africa, 2013. [Google Scholar]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and quantitative analysis of mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Sinha, K.K. Testing methods for aflatoxins in foods. Food Nutr. Bull. 1999, 20, 458–464. [Google Scholar] [CrossRef]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014. [Google Scholar] [CrossRef]
- Roseanu, A.; Jecu, L.; Badea, M.; Evans, R.W. Mycotoxins: An overview on their quantification methods. Rom. J. Biochem. 2010, 47, 79–86. [Google Scholar]
- Siwela, A.H.; Siwela, M.; Matindi, G.; Dube, S.; Nziramasanga, N. Decontamination of aflatoxin-contaminated maize by dehulling. J. Sci. Food Agric. 2005, 85, 2535–2538. [Google Scholar] [CrossRef]
- Mazumder, P.M.; Sasmal, D. Mycotoxins-limits and regulations. Anc. Sci. Life 2001, 20, 1. [Google Scholar] [PubMed]
- Pswarayi, F.; Mutukumira, A.N.; Chipurura, B.; Gabi, B.; Jukes, D.J. Food control in Zimbabwe: A situational analysis. Food Control 2014, 46, 143–151. [Google Scholar] [CrossRef]
- Mutukumira, A.; Jukes, D.J. The Development of National Food Safety Control Systems in Sub-Saharan Africa-Issues and Opportunities. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.543.4408&rep=rep1&type=pdf (accessed on 14 April 2018).
- Mutegi, C.; Wagacha, M.; Kimani, J.; Otieno, G.; Wanyama, R.; Hell, K.; Christie, M.E. Incidence of aflatoxin in peanuts (Arachis hypogaea linnaeus) from markets in western, Nyanza and Nairobi provinces of Kenya and related market traits. J. Stored Prod. Res. 2013, 52, 118–127. [Google Scholar] [CrossRef]
- Ogodo, A.C.; Ugbogu, O.C. Public health significance of aflatoxin in food industry—A review. Eur. J. Clin. Biom. Sci. 2016, 2, 51–58. [Google Scholar]
- Kamika, I.; Takoy, L.L. Natural occurrence of aflatoxin B1 in peanut collected from Kinshasa, Democratic Republic of Congo. Food Control 2011, 22, 1760–1764. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Galaverna, G.; Mangia, M.; Sforza, S.; Dossena, A.; Marchelli, R. Free and bound fumonisins in gluten-free food products. Mol. Nutr. Food Res. 2009, 53, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Katerere, D.R.; Shephard, G.S.; Faber, M. Infant malnutrition and chronic aflatoxicosis in southern Africa: Is there a link? Int. J. Food Saf. Nutr. Public Health 2008, 1, 127–136. [Google Scholar] [CrossRef]
- Loreen, D.; Moses, M. Assessment of aflatoxin awareness by players in groundnut value chain: The case of Dora in Mutare, Zimbabwe. Int. J. Innov. Res. Dev. 2015, 4, 90–99. [Google Scholar]
- Ezeokeke, C.T.; Onuoha, A.B. Nutrient composition of cereal (maize), legume (soybean) and fruit (banana) as a complementary food for older infants and their sensory assessment. J. Food Sci. Eng 2016, 6, 139–148. [Google Scholar]
- Fandohan, P.; Ahouansou, R.; Houssou, P.; Hell, K.; Marasas, W.; Wingfield, M. Impact of mechanical shelling and dehulling on Fusarium infection and fumonisin contamination in maize. Food Add. Contam. 2006, 23, 415–421. [Google Scholar] [CrossRef] [PubMed]
| Food/Feed | Mycotoxin | Sample Source | Concentration Range (µg/kg) | Analytical Method | Reference |
|---|---|---|---|---|---|
| Groundnut | Total AF | Food and feed companies | ˂1–394 | HPLC | [48] |
| Peanut butter | Total AF | Food and feed companies | ˂1–213 | HPLC | |
| Cowpeas | Total AF | Food and feed companies | ˂1–20 | HPLC | |
| Maize | Total AF | Food and feed companies | ˂1–1391 | HPLC | |
| Stock feed | Total AF | Food and feed companies | ˂1–30 | HPLC | |
| Cornstarch | Total AF | Food and feed companies | ˂1 | HPLC | |
| Beans | Total AF | Food and feed companies | ˂1–30 | HPLC | |
| Groundnuts | AFB1 | Grain marketing board | ˂5–250 | TLC | [28] |
| AFG1 | Grain marketing board | ˂4–200 | TLC | ||
| Groundnuts | Total AF | Retail shops and vendors | 6.6–622.1 | HPLC | [2] |
| Peanut butter | Total AF | Retail shops and vendors | 6.1–247 | HPLC | |
| Groundnuts | AFB1 | Makoni District | 0.7–108.3 | HPLC | [27] |
| AFB1 | Shamva District | 3.1–175.9 | HPLC | ||
| AFB2 | Makoni District | 1.3–320 | HPLC | ||
| AFB2 | Shamva District | 1.3–320 | HPLC | ||
| AFG1 | Makoni District | 20.9–271.6 | HPLC | ||
| AFG1 | Shamva District | 20.9–271.6 | HPLC | ||
| AFG2 | Makoni District | 29.1–377.8 | HPLC | ||
| AFG2 | Shamva District | 29.1–377.8 | HPLC | ||
| Total AF | Makoni District | 9.2–697.8 | HPLC | ||
| Total AF | Shamva District | 9.2–697.8 | HPLC | ||
| Beans | Total AF | Shamva District | 27.3 | HPLC | [27] |
| Total AF | Makoni District | 70.9 | HPLC | ||
| Cowpeas | Total AF | Makoni District | 1.4–103.4 | HPLC | |
| Total AF | Shamva District | 2.3 | HPLC | ||
| Bambara nuts | Total AF | Makoni District | 8.6–53.5 | HPLC | |
| Stink bugs | AFB1 | Bikita District | 0.5–0.59 | LC-QtoF-MS | [49] |
| Milk | AFM1 | Harare | 590–4510 * | HPLC | [50] |
| Maize | Total AF | Zimbabwe | ˂1–123 | ELISA | [46] |
| Maize | AFB1 | Zimbabwe | ˂1–11 | LC-MS/MS | [51] |
| Maize | AFB1 | Shamva District | 0.65–26.65 | ELISA | [17] |
| Makoni District | 0.57–9.22 | ELISA | |||
| Maize | AFG1 | Manicaland and Mashonaland Provinces | nd–23.7 | HPLC | [20] |
| Maize stovers | AFB1 | Silozwi Communal Area | 0.8–1.6 | HPLC | [52] |
| Food/Feed | Mycotoxin | Sample Source | Concentration Range (µg/kg) | Analytical Method | Reference |
|---|---|---|---|---|---|
| Maize | Fumonisins | Zimbabwe | 36,000–159,000 | ELISA | [46] |
| DON | nd–12,000 | ELISA | |||
| Maize | FB1 | Zimbabwe | nd–1106 | LC-MS/MS | [67] |
| DON | nd–492 | LC-MS/MS | |||
| Sorghum | Fumonisins | Zimbabwe | 8–187 | HPLC | [68] |
| Maize | FB1 | Shamva District | 108.35–337.14 | ELISA | [17] |
| Makoni District | 15.65–579.6 | ELISA | |||
| Maize meal | FB1 | Shamva District | 10.43–432.32 | ELISA | |
| Makoni District | 13.84–606.64 | ELISA | |||
| Maize (preharvest) | DON | Mainacland and Mashonaland West Provinces | nd–220.6 | HPLC | [20] |
| ZEA | nd–44.6 | HPLC | |||
| FB1 | nd–3865.99 | HPLC | |||
| FB2 | nd–819.01 | HPLC | |||
| FB3 | nd–472.20 | HPLC | |||
| Maize (at harvest) | DON | Mainacland and Mashonaland West Provinces | nd–184 | HPLC | |
| ZEA | nd–16.3 | HPLC | |||
| FB1 | nd–3866 | HPLC | |||
| FB2 | nd–431 | HPLC | |||
| FB3 | nd–472 | HPLC | |||
| Maize (3 months in storage) | DON | Mainacland and Mashonaland West Provinces | nd–147 | HPLC | [20] |
| ZEA | nd–30.8 | HPLC | |||
| FB1 | nd–2842 | HPLC | |||
| FB2 | nd–819 | HPLC | |||
| FB3 | nd–275 | HPLC | |||
| Maize (6 months in storage) | DON | Mainacland and Mashonaland West Provinces | nd–115 | HPLC | |
| ZEA | nd–44.6 | HPLC | |||
| FB1 | nd–2709 | HPLC | |||
| FB2 | nd–424 | HPLC | |||
| FB3 | nd–121 | HPLC | |||
| Maize stovers (beginning of storage) | FB1 | Silozwi Communal Area | 109–752 | HPLC | [52] |
| ZEA | nd–73 | ||||
| Maize stovers (end of storage, i.e., 4 months later) | FB1 | Silozwi Communal Area | nd–38 | HPLC |
| Sample | Mycotoxin | Analytical Method | References |
|---|---|---|---|
| Groundnuts | Total Aflatoxin | TLC | [28] |
| HPLC | [27] | ||
| Maize | Fumonisins | HPLC | [21,73] |
| AFB1 | HPLC | [20] | |
| AFB2 | LC-MS/MS | ||
| AFG1 | LC-MS/MS | ||
| AFG2 | LC-MS/MS | ||
| FB1 | LC-MS/MS | ||
| FB2 | LC-MS/MS | ||
| FB3 | LC-MS/MS | ||
| ZEA | LC-MS/MS | ||
| DON | LC-MS/MS | ||
| OTA | LC-MS/MS | [17] | |
| AFB1 | HPLC | ||
| FB1 | ELISA | ||
| Urine | AFM1 | ELISA | [43] |
| TLC, HPLC | [44] | ||
| Peanut butter | Total aflatoxin | HPLC | [55] |
| Beans | Total Aflatoxin | HPLC | [27] |
| Cowpeas | Total Aflatoxin | HPLC | |
| Bambara nuts | Total Aflatoxin | HPLC | |
| Milk | AFM1 | HPLC | [50] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nleya, N.; Adetunji, M.C.; Mwanza, M. Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe. Toxins 2018, 10, 89. https://doi.org/10.3390/toxins10050089
Nleya N, Adetunji MC, Mwanza M. Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe. Toxins. 2018; 10(5):89. https://doi.org/10.3390/toxins10050089
Chicago/Turabian StyleNleya, Nancy, Modupeade Christianah Adetunji, and Mulunda Mwanza. 2018. "Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe" Toxins 10, no. 5: 89. https://doi.org/10.3390/toxins10050089
APA StyleNleya, N., Adetunji, M. C., & Mwanza, M. (2018). Current Status of Mycotoxin Contamination of Food Commodities in Zimbabwe. Toxins, 10(5), 89. https://doi.org/10.3390/toxins10050089