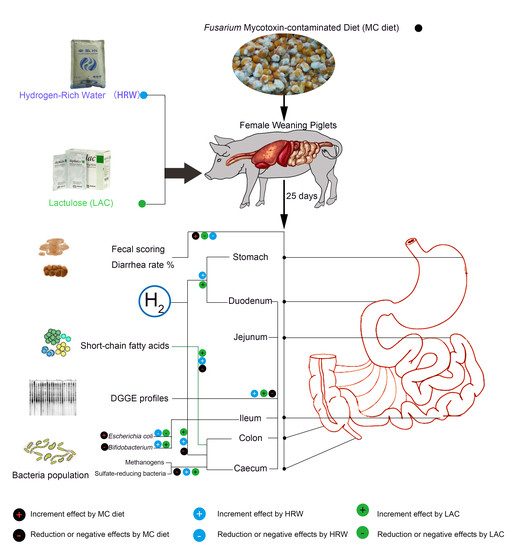
Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet
Abstract
:1. Introduction
2. Results
2.1. Hydrogen Levels in Intestinal Segments
2.2. Fecal Scoring and Diarrhea Rate
2.3. Short-Chain Fatty Acids (SCFAs) Levels in the Digesta of Jejumun, Ileum, Colon, and Caecum
2.4. Microbiota Communities of Total Bacteria and Methanogenic Archaea in the Digesta of Intestinal Segments
2.5. Populations of Selected Bacteria in the Digesta of Different Intestinal Segments
3. Discussion
3.1. Hydrogen Levels in Intestinal Segments
3.2. Fecal Scoring and Diarrhea Rate
3.3. SCFAs Levels in the Digesta of Jejumun, Ileum, Colon, and Caecum
3.4. Microbiota Communities and Populations
4. Conclusions
5. Materials and Methods
5.1. Preparation of Fusarium Mycotoxin-Contaminated Maize
5.2. Experimental Diets and Mycotoxins Analyusis
5.3. Animals
5.4. Experimental Design and Sampling
5.5. Feces Scoring
5.6. Hydrogen Gas Measurement in Different Intestine Segments
5.7. SCFA Detection in the Digesta of Different Intestine Segements
5.8. DNA Isolation, PCR Amplification, and DGGE Analysis
5.9. Real-Time PCR Assays for Quantification of the Selected Bacteria
5.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cortinovis, C.; Pizzo, F.; Spicer, L.J.; Caloni, F. Fusarium mycotoxins: Effects on reproductive function in domestic animals—A review. Theriogenology 2013, 80, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Danicke, S. The fusarium toxins deoxynivalenol (don) and zearalenone (zon) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kollarczik, B.; Gareis, M.; Hanelt, M. In vitro transformation of the fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Nat. Toxins 1994, 2, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Karlovsky, P. Biological detoxification of the mycotoxin deoxynivalenol and its use in genetically engineered crops and feed additives. Appl. Microbiol. Biotechnol. 2011, 91, 491–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wache, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Slizewska, K.; Nowak, A.; Zielonka, L.; Zakowska, Z.; Gajecka, M.; Gajecki, M. The effect of experimental fusarium mycotoxicosis on microbiota diversity in porcine ascending colon contents. Toxins 2014, 6, 2064–2081. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Yang, M.; Yang, N.N.; Yin, X.X.; Song, W.G. Molecular hydrogen: A preventive and therapeutic medical gas for various diseases. Oncotarget 2017, 8, 102653–102673. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kajiya, M.; Silva, M.J.B.; Sato, K.; Ouhara, K.; Kawai, T. Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. BBRC 2009, 386, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Mao, Y.; Cai, J.; Li, Y.; Liu, W.; Sun, P.; Zhang, J.H.; Sun, X.; Yuan, H. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic. Res. 2009, 43, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.G.; Biswas, A.; Morales, S.E.; Greening, C.; Gaskins, H.R. H2 metabolism is widespread and diverse among human colonic microbes. Gut Microbes 2016, 7, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, Y.; Shinke, M.; Hiraishi, M.; Tsuchiya, Y.; Masuda, S. The application of alkaline and acidic electrolyzed water in the sterilization of chicken breasts and beef liver. Food Sci. Nutr. 2016, 4, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.W.; Li, Y.; Luo, D.; Dong, J.L.; Zhou, L.X.; Zhao, S.Y.; Zheng, Q.S.; Wang, H.C.; Cui, M.; Fan, S.J. Hydrogen-water ameliorates radiation-induced gastrointestinal toxicity via myd88’s effects on the gut microbiota. Exp. Mol. Med. 2018, 50, e433. [Google Scholar] [CrossRef] [PubMed]
- Panesar, P.S.; Kumari, S. Lactulose: Production, purification and potential applications. Biotechnol. Adv. 2011, 29, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, W.; Zhang, R.; Ruan, X.; Ren, P.; Lu, B. Lactulose accelerates liver regeneration in rats by inducing hydrogen. J. Surg. Res. 2015, 195, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating nrf2 expression. Free Radic. Biol. Med. 2013, 65, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Chae, J.P.; Pajarillo, E.A.B.; Park, C.-S.; Kang, D.-K. Lactulose increases bacterial diversity and modulates the swine faecal microbiome as revealed by 454-pyrosequencing. Anim. Feed Sci. Technol. 2015, 209, 157–166. [Google Scholar] [CrossRef]
- Zheng, W.; Hou, Y.; Su, Y.; Yao, W. Lactulose promotes equol production and changes the microbial community during in vitro fermentation of daidzein by fecal inocula of sows. Anaerobe 2014, 25, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sommer, F.; Backhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Wang, Y.P.; Liu, S.Y.; Huang, J.J.; Zhai, Z.X.; He, C.; Ding, J.M.; Wang, J.; Wang, H.J.; Fan, W.B.; et al. The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Kamimura, N.; Iuchi, K.; Nishimaki, K.; Yokota, T.; Ogawa, R.; Ohta, S. Protective effect of hydrogen gas inhalation on muscular damage using a mouse hindlimb ischemia-reperfusion injury model. Plast. Reconstr. Surg. 2017, 140, 1195–1206. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, S.; Zhang, J. Hydrogen as a selective antioxidant: A review of clinical and experimental studies. J. Int. Med. Res. 2010, 38, 1893–1903. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuo, Q.; Hai, Y.; Sun, X.J. Lactulose: An indirect antioxidant ameliorating inflammatory bowel disease by increasing hydrogen production. Med. Hypotheses 2011, 76, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, X.; Shi, J.; Liu, W.W.; Tao, H.; Sun, X.; Kang, Z. Lactulose mediates suppression of dextran sodium sulfate-induced colon inflammation by increasing hydrogen production. Dig. Dis. Sci. 2013, 58, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Daga, A.; Horn, M.B.; Kottwitz, L.B.M.; de Farina, L.O. Bromatological and mycotoxin analysis on soybean meal before and after the industrial process of micronization. Cienc. Rural 2015, 45, 1336–1341. [Google Scholar] [CrossRef] [Green Version]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Ji, X.; Zhang, Q.; Du, W.; Wei, Q.; Yao, W. Hydrogen-rich water and lactulose protect against growth suppression and oxidative stress in female piglets fed fusarium toxins contaminated diets. Toxins 2018, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Gahan, D.A.; Lynch, M.B.; Callan, J.J.; O’Sullivan, J.T.; O’Doherty, J.V. Performance of weanling piglets offered low-, medium- or high-lactose diets supplemented with a seaweed extract from laminaria spp. Animal 2009, 3, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Krueger, M.; Schroedl, W.; Isik, W.; Lange, W.; Hagemann, L. Effects of lactulose on the intestinal microflora of periparturient sows and their piglets. Eur. J. Nutr. 2002, 41 (Suppl. 1), I26–I31. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ordaz, A.A.; Molist, F.; Hermes, R.G.; de Segura, A.G.; La Ragione, R.M.; Woodward, M.J.; Tchorzewska, M.A.; Collins, J.W.; Pérez, J.F.; Martín-Orúe, S.M. Effect of inclusion of lactulose and lactobacillus plantarum on the intestinal environment and performance of piglets at weaning. Anim. Feed Sci. Technol. 2013, 185, 160–168. [Google Scholar] [CrossRef]
- Richards, J.D.; Gong, J.; de Lange, C.F.M. The gastrointestinal microbiota and its role in monogastric nutrition and health with an emphasis on pigs: Current understanding, possible modulations, and new technologies for ecological studies. Can. J. Anim. Sci. 2005, 85, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Kamphues, J.; Tabeling, R.; Stuke, O.; Bollmann, S.; Amtsberg, G. Investigations on potential dietetic effects of lactulose in pigs. Livest. Sci. 2007, 109, 93–95. [Google Scholar] [CrossRef]
- Calik, A.; Ergun, A. Effect of lactulose supplementation on growth performance, intestinal histomorphology, cecal microbial population, and short-chain fatty acid composition of broiler chickens. Poult. Sci. 2015, 94, 2173–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martín-Peláez, S.; Costabile, A.; Hoyles, L.; Rastall, R.A.; Gibson, G.R.; La Ragione, R.M.; Woodward, M.J.; Mateu, E.; Martín-Orúe, S.M. Evaluation of the inclusion of a mixture of organic acids or lactulose into the feed of pigs experimentally challenged with salmonella typhimurium. Vet. Microbiol. 2010, 142, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Danicke, S.; Matthaus, K.; Lebzien, P.; Valenta, H.; Stemme, K.; Ueberschar, K.H.; Razzazi-Fazeli, E.; Bohm, J.; Flachowsky, G. Effects of fusarium toxin-contaminated wheat grain on nutrient turnover, microbial protein synthesis and metabolism of deoxynivalenol and zearalenone in the rumen of dairy cows. J. Anim. Physiol. Anim. Nutr. 2005, 89, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Ali-Vehmas, T.; Rizzo, A.; Westermarck, T.; Atroshi, F. Measurement of antibacterial activities of t-2 toxin, deoxynivalenol, ochratoxin a, aflatoxin b1 and fumonisin b1 using microtitration tray-based turbidimetric techniques. Zentralbl. Veterinarmed. A 1998, 45, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Burel, C.; Tanguy, M.; Guerre, P.; Boilletot, E.; Cariolet, R.; Queguiner, M.; Postollec, G.; Pinton, P.; Salvat, G.; Oswald, I.P.; et al. Effect of low dose of fumonisins on pig health: Immune status, intestinal microbiota and sensitivity to salmonella. Toxins 2013, 5, 841–864. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Ordaz, A.A.; Gonzalez-Ortiz, G.; La Ragione, R.M.; Woodward, M.J.; Collins, J.W.; Perez, J.F.; Martin-Orue, S.M. Lactulose and lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl. Environ. Microbiol. 2014, 80, 4879–4886. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Higashimura, Y.; Baba, Y.; Inoue, R.; Takagi, T.; Uchiyama, K.; Mizushima, K.; Hirai, Y.; Ushiroda, C.; Tanaka, Y. Effects of molecular hydrogen-dissolved alkaline electrolyzed water on intestinal environment in mice. Med. Gas Res. 2018, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Duan, Y.; Ge, C.; Chen, C.; Zhou, M. Functional analysis of the beta2-tubulin gene of fusarium graminearum and the beta-tubulin gene of botrytis cinerea by homologous replacement. Pest Manag. Sci. 2013, 69, 582–588. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (U.S.). Committee on Nutrient Requirements of Swine. In Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Xiao, H.; Wu, M.M.; Tan, B.E.; Yin, Y.L.; Li, T.J.; Xiao, D.F.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.T.; Xie, W.M.; Zhang, N.Y.; Dai, J.F.; Wang, Y.; Rajput, S.A.; Qi, D.S.; et al. Individual and combined occurrence of mycotoxins in feed ingredients and complete feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.M.; Sweeney, T.; O’Shea, C.J.; Doyle, D.N.; O’Doherty, J.V. Effect of supplementing different ratios of laminarin and fucoidan in the diet of the weanling piglet on performance, nutrient digestibility, and fecal scoring. J. Anim. Sci. 2012, 90, 215–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Chen, L.; Zhou, W.; Hu, L.; Li, L.; Tu, Q.; Chang, Y.; Liu, Q.; Sun, X.; Wu, M.; et al. The protective role of hydrogen-rich saline in experimental liver injury in mice. J. Hepatol. 2011, 54, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.Y.; Zhu, W.Y.; Wang, Q.J.; Yao, W. Effect of daidzein on in vitro fermentation by microorganisms from the goat rumen Anim. Feed Sci. Technol. 2007, 136, 154–163. [Google Scholar] [CrossRef]
- Zheng, W.; Hou, Y.; Yao, W. Lactulose increases equol production and improves liver antioxidant status in barrows treated with daidzein. PLoS ONE 2014, 9, e93163. [Google Scholar] [CrossRef] [PubMed]




| Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| Fecal score | ||||||
| Days 0–7 | 2.64 c | 3.60 a | 3.17 b | 2.64 c | 0.10 | <0.001 |
| Days 7–14 | 2.84 b | 3.74 a | 3.12 b | 3.02 b | 0.12 | 0.037 |
| Days 14–21 | 2.78 | 3.00 | 2.82 | 2.95 | 0.11 | 0.909 |
| Days 21–25 | 2.79 | 2.53 | 2.82 | 3.00 | 0.15 | 0.758 |
| Days 0–25 | 2.76 | 3.30 | 3.02 | 2.91 | 0.09 | 0.163 |
| Diarrhea rate % | ||||||
| Days 0–7 | 5.00 b | 30.00 a | 25.00 a | 5.00 b | 3.64 | 0.008 |
| Days 7–14 | 5.00 | 30.00 | 22.50 | 15.00 | 5.00 | 0.353 |
| Days 14–21 | 7.50 | 17.50 | 15.00 | 12.50 | 5.09 | 0.927 |
| Days 21–25 | 12.00 | 0.00 | 12.00 | 16.00 | 5.53 | 0.788 |
| Days 0–25 | 6.92 | 22.31 | 20.00 | 12.31 | 3.65 | 0.449 |
| Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|
| Jejunum (μmol/g wt digesta) | ||||||
| Acetate | 4.30 | 3.80 | 4.48 | 4.14 | 0.27 | 0.819 |
| Propionate | 2.08 | 1.58 | 1.83 | 1.99 | 0.12 | 0.486 |
| Butyrate | 0.94 | 0.72 | 0.84 | 1.00 | 0.06 | 0.425 |
| Total SCFAs | 7.45 | 6.10 | 7.15 | 7.13 | 0.43 | 0.737 |
| Ileum (μmol/g wt digesta) | ||||||
| Acetate | 10.50 | 10.64 | 10.58 | 10.29 | 0.41 | 0.993 |
| Propionate | 5.09 | 5.56 | 5.65 | 5.16 | 0.27 | 0.660 |
| Butyrate | 0.49 | 0.50 | 0.52 | 0.54 | 0.03 | 0.932 |
| Total SCFAs | 16.08 | 16.69 | 16.75 | 15.99 | 0.66 | 0.971 |
| Colon (μmol/g wt digesta) | ||||||
| Acetate | 44.28 | 39.83 | 49.94 | 43.78 | 1.48 | 0.102 |
| Propionate | 19.13 a | 11.19 b | 20.13 a | 14.62 ab | 1.17 | 0.010 |
| Butyrate | 8.25 a | 4.00 b | 7.55 a | 7.35 a | 0.53 | 0.008 |
| Valeric acid | 4.40 a | 1.00 c | 2.55 b | 0.91 c | 0.40 | <0.001 |
| Total SCFAs | 76.06 a | 56.02 b | 80.18 a | 66.66 ab | 2.99 | 0.008 |
| Caecum (μmol/g wt digesta) | ||||||
| Acetate | 57.29 a | 51.47 b | 60.45 a | 60.91 a | 1.14 | 0.003 |
| Propionate | 26.20 a | 19.12 b | 25.97 a | 22.50 ab | 0.99 | 0.020 |
| Butyrate | 12.43 a | 6.02 c | 9.89 b | 10.04 b | 0.59 | <0.001 |
| Valeric acid | 9.84 a | 2.49 b | 2.22 b | 3.01 b | 0.77 | <0.001 |
| Total SCFAs | 105.76 a | 79.10 c | 98.53 ab | 96.46 b | 2.60 | <0.001 |
| Target Group | DNA Sample | Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|---|---|
| Total bacteria | Jejunum | Band number | 59.20 | 60.60 | 64.60 | 59.60 | 1.34 | 0.498 |
| Shannon diversity | 3.40 | 3.64 | 3.68 | 3.60 | 0.05 | 0.278 | ||
| Ileum | Band number | 34.40 c | 37.70 bc | 44.40 a | 38.40 b | 1.00 | <0.001 | |
| Shannon diversity | 3.00 b | 3.27 a | 3.49 a | 3.34 a | 0.06 | 0.006 | ||
| Colon | Band number | 40.00 b | 50.40 a | 52.00 a | 40.00 b | 1.60 | 0.001 | |
| Shannon diversity | 3.14 | 3.15 | 3.14 | 3.19 | 0.04 | 0.948 | ||
| Caecum | Band number | 61.60 b | 72.60 a | 72.80 a | 71.60 a | 4.23 | 0.036 | |
| Shannon diversity | 3.55 | 3.76 | 3.75 | 3.81 | 0.04 | 0.070 | ||
| Methanogens | Ileum | Band number | 15.20 b | 26.80 a | 17.20 b | 18.40 b | 1.14 | <0.001 |
| Shannon diversity | 2.27 b | 2.91 a | 2.27 b | 2.28 b | 0.09 | 0.015 | ||
| Colon | Band number | 29.80 bc | 36.20 a | 32.20 b | 27.40 c | 0.89 | <0.001 | |
| Shannon diversity | 2.99 | 3.23 | 2.87 | 2.89 | 0.07 | 0.208 | ||
| Caecum | Band number | 31.40 b | 41.00 a | 32.80 b | 34.60 b | 1.27 | 0.023 | |
| Shannon diversity | 3.06 | 3.34 | 2.92 | 3.22 | 0.08 | 0.234 |
| Sample | Item | NC | MC | MC + LAC | MC + HRW | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Jejunum | All bacteria | 8.75 | 8.66 | 8.67 | 8.20 | 0.13 | 0.486 |
| Lactobacillus | 7.96 | 7.43 | 7.25 | 7.28 | 0.19 | 0.552 | |
| Bifidobacterium | 4.90 | 4.76 | 5.26 | 5.14 | 0.08 | 0.124 | |
| Escherichia coli | 4.87 | 5.78 | 5.53 | 5.31 | 0.14 | 0.127 | |
| Enterococcus | 3.30 | 3.45 | 3.21 | 3.06 | 0.11 | 0.685 | |
| Ileum | All bacteria | 9.04 | 9.64 | 9.43 | 9.53 | 0.14 | 0.221 |
| Lactobacillus | 8.85 | 8.60 | 8.75 | 8.86 | 0.25 | 0.906 | |
| Bifidobacterium | 5.07 a | 4.23 b | 5.26 a | 5.13 a | 0.13 | 0.009 | |
| Escherichia coli | 5.67 b | 7.25 a | 6.14 b | 6.21 b | 0.16 | 0.005 | |
| Enterococcus | 3.08 | 3.66 | 3.39 | 3.32 | 0.58 | 0.488 | |
| Colon | All bacteria | 11.04 | 11.09 | 11.17 | 10.93 | 0.07 | 0.704 |
| Methanogens | 4.49 a | 3.47 c | 5.52 a | 4.58 b | 0.20 | 0.001 | |
| SRB 3 | 4.54 a | 3.27 b | 4.68 a | 4.12 a | 0.18 | 0.011 | |
| Acetogenic bacteria | 6.87 | 6.85 | 7.17 | 6.54 | 0.11 | 0.297 | |
| Caecum | All bacteria | 11.57 | 11.34 | 11.69 | 11.60 | 0.05 | 0.119 |
| Methanogens | 5.14 | 5.55 | 5.44 | 5.36 | 0.28 | 0.969 | |
| SRB 3 | 2.89 | 3.39 | 3.02 | 2.88 | 0.12 | 0.470 | |
| Acetogenic bacteria | 6.21 | 6.31 | 6.32 | 6.19 | 0.16 | 0.992 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, W.; Ji, X.; Zhang, Q.; Yao, W. Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet. Toxins 2018, 10, 246. https://doi.org/10.3390/toxins10060246
Zheng W, Ji X, Zhang Q, Yao W. Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet. Toxins. 2018; 10(6):246. https://doi.org/10.3390/toxins10060246
Chicago/Turabian StyleZheng, Weijiang, Xu Ji, Qing Zhang, and Wen Yao. 2018. "Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet" Toxins 10, no. 6: 246. https://doi.org/10.3390/toxins10060246
APA StyleZheng, W., Ji, X., Zhang, Q., & Yao, W. (2018). Intestinal Microbiota Ecological Response to Oral Administrations of Hydrogen-Rich Water and Lactulose in Female Piglets Fed a Fusarium Toxin-Contaminated Diet. Toxins, 10(6), 246. https://doi.org/10.3390/toxins10060246