A Sensitive, Point-of-Care Detection of Small Molecules Based on a Portable Barometer: Aflatoxins In Agricultural Products
Abstract
:1. Introduction
2. Results and Discussion
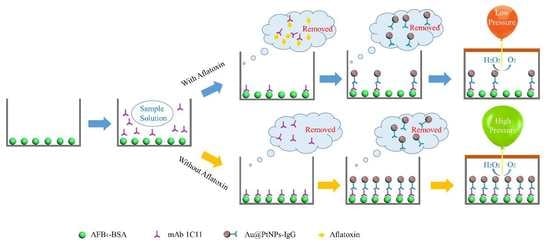
2.1. Principle of POCAD
2.2. Characterization of Au@PtNPs
2.3. Synthesis of Au@PtNPs-IgG
2.4. Characterization of Au@PtNPs-IgG
2.5. Optimization of POCAD
2.5.1. Blocking Solution for Immunoassay
2.5.2. Methanol Concentration for Immunoassay
2.6. Standard Curve and LOD
2.7. Recovery
2.8. Within Assay and between Assay
2.9. Validation via HPLC Using Peanut and Corn Samples
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. Synthesis and Characterization of Au@PtNPs-IgG
4.3. Fabrication of POCAD
4.4. Procedure of POCAD
4.5. Optimization of POCAD
4.6. Evaluation of POCAD
4.6.1. LOD and Linear Range
4.6.2. Recovery, Inner-Assay Precision and Inter-Assay Precision
4.7. Validation via Immune Affinity Column-HPLC
4.8. Sample Pretreatment
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.J.; Ryu, D. Worldwide occurrence of mycotoxins in cereals and cereal-derived food products: Public health perspectives of their co-occurrence. J. Agric. Food Chem. 2017, 65, 7034–7051. [Google Scholar] [CrossRef] [PubMed]
- Nesbitt, B.F.; O’Kelly, J.; Sargeant, K.; Sheridan, A. Aspergillus flavus and turkey X disease. Toxic metabolites of aspergillus flavus. Nature 1962, 195, 1062–1063. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, S28–S48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, S.H.; Bosch, F.X.; Troxell, T.C.; Bolger, P.M. Public health—Reducing liver cancer—Global control of aflatoxin. Science 1999, 286, 2453–2454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shephard, G.S. Aflatoxin analysis at the beginning of the twenty-first century. Anal. Bioanal. Chem. 2009, 395, 1215–1224. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, H.; Yan, X.; Peng, H.; Wang, Z.; Zhang, Q.; Li, P.; Zhang, Z.; Le, X.C. Binding-induced DNA dissociation assay for small molecules: Sensing aflatoxin B1. ACS Sens. 2018, 3, 2590–2596. [Google Scholar] [CrossRef]
- Yu, L.; Ma, F.; Ding, X.X.; Wang, H.L.; Li, P.W. Silica/graphene oxide nanocomposites: Potential adsorbents for solid phase extraction of trace aflatoxins in cereal crops coupled with high performance liquid chromatography. Food Chem. 2018, 245, 1018–1024. [Google Scholar] [CrossRef]
- McCullum, C.; Tchounwou, P.; Ding, L.-S.; Liao, X.; Liu, Y.-M. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for Hplc-Ms/Ms analysis. J. Agric. Food Chem. 2014, 62, 4261–4267. [Google Scholar] [CrossRef]
- Liu, B.-H.; Chu, K.-C.; Yu, F.-Y. Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Li, Y.; Xuan, J.; Song, Y.J.; Qi, W.J.; He, B.S.; Wang, P.; Qin, L.D. Nanoporous glass integrated in volumetric bar-chart chip for point-of-care diagnostics of non-small cell lung cancer. ACS Nano 2016, 10, 1640–1647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.L.; Mao, Y.; Huang, D.; He, Z.; Yan, J.M.; Tian, T.; Shi, Y.Z.; Song, Y.L.; Li, X.R.; Zhu, Z.; et al. Portable visual quantitative detection of aflatoxin B-1 using a target-responsive hydrogel and a distance-readout microfluidic chip. Lab Chip 2016, 16, 3097–3104. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jia, S.S.; Zhang, H.M.; Ma, Y.L.; Guan, Z.C.; Li, J.X.; Zhu, Z.; Ji, T.H.; Yang, C.J. Integrating target-responsive hydrogel with pressuremeter readout enables simple, sensitive, user-friendly, quantitative point-of-care testing. ACS Appl. Mater. Interfaces 2017, 9, 22252–22258. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tian, T.; Chen, X.; Lei, Z.; Song, Y.; Shi, Y.; Ji, T.; Zhu, Z.; Yang, L.; Yang, C. Gas-generating reactions for point-of-care testing. Analyst 2018, 143, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Engelbrekt, C.; Seselj, N.; Poreddy, R.; Riisager, A.; Ulstrup, J.; Zhang, J. Atomically thin Pt shells on Au nanoparticle cores: Facile synthesis and efficient synergetic catalysis. J. Mater. Chem. A 2016, 4, 3278–3286. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Lin, B.; Song, Y.; Guan, Z.; Cheng, J.; Zhu, Z.; Yang, C. Staining traditional colloidal gold test strips with Pt nanoshell enables quantitative point-of-care testing with simple and portable pressure meter readout. ACS Appl. Mater. Interfaces 2019, 11, 1800–1806. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.B.; Maye, M.M.; Han, L.; Luo, J.; Zhong, C.J. Gold-platinum alloy nanoparticle assembly as catalyst for methanol electrooxidation. Chem. Commun. 2001, 5, 473–474. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, Z.; Li, J.; Wu, Z.; Zhong, H.; Yang, Q.; Liu, Q.; Liu, Z.; Sheng, L.; Xu, M.; et al. Quantitative assessment of disease markers using the naked eye: Point-of-care testing with gas generation-based biosensor immunochromatographic strips. J. Nanobiotechnol. 2019, 17, 67. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Z.; Jia, S.; Lei, Z.; Lin, S.; Zhang, H.; Ma, Y.; Tian, Z.-Q.; Yang, C.J. Au@Pt nanoparticle encapsulated target-responsive hydrogel with volumetric bar-chart chip readout for quantitative point-of-care testing. Angew. Chem. Int. Ed. 2014, 53, 12503–12507. [Google Scholar]
- Huang, Y.; Ma, Y.; Chen, Y.; Wu, X.; Fang, L.; Zhu, Z.; Yang, C.J. Target-responsive dnazyme cross-linked hydrogel for visual quantitative detection of lead. Anal. Chem. 2014, 86, 11434–11439. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Liu, X.L.; Zheng, X.H.; Zheng, J.B. Synthesis of Au@Pt nanoflowers supported on graphene oxide for enhanced electrochemical sensing of dopamine. J. Electroanal. Chem. 2018, 817, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zheng, X.; Zheng, J. Facile synthesis of three-dimensional porous Au@Pt core-shell nanoflowers supported on graphene oxide for highly sensitive and selective detection of hydrazine. Chem. Eng. J. 2017, 327, 431–440. [Google Scholar] [CrossRef]
- Peng, C.F.; Pan, N.; Qian, Z.J.; Wei, X.L.; Shao, G. Colorimetric detection of thiocyanate based on inhibiting the catalytic activity of cystine-capped core-shell Au@Pt nanocatalysts. Talanta 2017, 175, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. Europium nanospheres-based time-resolved fluorescence for rapid and ultrasensitive determination of total aflatoxin in feed. J. Agric. Food Chem. 2015, 63, 10313–10318. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Chen, M.; Wang, S.; Liu, K.; Lai, W. Rapid detection method for aflatoxin B-1 in Soybean sauce based on fluorescent microspheres probe. Food Control 2015, 50, 659–662. [Google Scholar] [CrossRef]
- Yu, L.; Li, P.; Ding, X.; Zhang, Q. Graphene oxide and carboxylated graphene oxide: Viable two-dimensional nanolabels for lateral flow immunoassays. Talanta 2017, 165, 167–175. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, G.; Huang, Y.; Yang, S.; Ren, S.; Gao, Z.; Chen, A. Dual-competitive lateral flow aptasensor for detection of aflatoxin B-1 in food and feedstuffs. J. Hazard. Mater. 2018, 344, 249–257. [Google Scholar] [CrossRef]
- Zhang, D.H.; Li, P.W.; Zhang, Q.; Zhang, W.; Huang, Y.L.; Ding, X.X.; Jiang, J. Production of ultrasensitive generic monoclonal antibodies against major aflatoxins using a modified two-step screening procedure. Anal. Chim. Acta 2009, 636, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, P.; Zhang, Q.; Li, R.; Zhang, W.; Zhang, Z.; Ding, X.; Tang, X. Multi-component immunochromatographic assay for simultaneous detection of aflatoxin B-1, ochratoxin A and zearalenone in agro-food. Biosens. Bioelectron. 2013, 49, 426–432. [Google Scholar] [CrossRef]
- Liu, Z.J.; Zhang, B.; Sun, J.F.; Yi, Y.H.; Li, M.; Du, D.L.; Zhu, F.; Luan, J.W. Highly efficient detection of salbutamol in environmental water samples by an enzyme immunoassay. Sci. Total Environ. 2018, 613, 861–865. [Google Scholar] [CrossRef]
- Hu, X.F.; Hu, R.; Zhang, Z.W.; Li, P.W.; Zhang, Q.; Wang, M. Development of a multiple immunoaffinity column for simultaneous determination of multiple mycotoxins in feeds using Uplc-Ms/Ms. Anal. Bioanal. Chem. 2016, 408, 6027–6036. [Google Scholar] [CrossRef] [PubMed]








| Assay Methods | Assay Target | LOD (ng/mL) | Reference |
|---|---|---|---|
| Europium nanospheres-based time-resolved fluorescence immunoassay | AFTs1 | 0.16 | [24] |
| Fluorescent microspheres-based test strip | AFB1 | 2.5 | [25] |
| Graphene oxide and carboxylated graphene oxide-based test strip | AFB1 | 0.3 | [26] |
| Aptamer based test strip | AFB1 | 0.1 | [27] |
| POCAD | AFTs | 0.03 | This work |
| Spike Level (μg/kg) | Mean ± SD | Average Recovery (%) | Relative Standard Deviation (RSD%) | |
|---|---|---|---|---|
| Within assay (n = 3)a | 5 | 4.25 ± 0.25c | 85.0 | 5.8 |
| 15 | 16.79 ± 0.72 | 112.0 | 4.3 | |
| 20 | 19.33 ± 0.52 | 96.7 | 2.7 | |
| Between assay (n = 5)b | 5 | 4.15 ± 0.16 | 83.1 | 3.8 |
| 15 | 14.87 ± 0.34 | 99.1 | 2.3 | |
| 20 | 17.68 ± 1.13 | 88.4 | 6.4 |
| Samples | This Work (μg/kg n = 3)a | HPLC (μg/kg n = 3) |
|---|---|---|
| 1 | 4.86 ±0.34b | 4.87 ± 0.08 |
| 2 | 15.10 ± 0.50 | 15.04 ± 0.40 |
| 3 | 22.51 ± 0.95 | 21.64 ± 0.25 |
| 4 | 4.22 ± 0.26 | 4.13 ± 0.10 |
| 5 | 13.38 ± 0.52 | 14.01 ± 0.17 |
| 6 | 18.98 ± 0.95 | 19.62 ± 0.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wu, W.; Cai, C.; Hu, X.; Li, H.; Bai, Y.; Zhang, Z.; Li, P. A Sensitive, Point-of-Care Detection of Small Molecules Based on a Portable Barometer: Aflatoxins In Agricultural Products. Toxins 2020, 12, 158. https://doi.org/10.3390/toxins12030158
Zhang W, Wu W, Cai C, Hu X, Li H, Bai Y, Zhang Z, Li P. A Sensitive, Point-of-Care Detection of Small Molecules Based on a Portable Barometer: Aflatoxins In Agricultural Products. Toxins. 2020; 12(3):158. https://doi.org/10.3390/toxins12030158
Chicago/Turabian StyleZhang, Weiqi, Wenqin Wu, Chong Cai, Xiaofeng Hu, Hui Li, Yizhen Bai, Zhaowei Zhang, and Peiwu Li. 2020. "A Sensitive, Point-of-Care Detection of Small Molecules Based on a Portable Barometer: Aflatoxins In Agricultural Products" Toxins 12, no. 3: 158. https://doi.org/10.3390/toxins12030158
APA StyleZhang, W., Wu, W., Cai, C., Hu, X., Li, H., Bai, Y., Zhang, Z., & Li, P. (2020). A Sensitive, Point-of-Care Detection of Small Molecules Based on a Portable Barometer: Aflatoxins In Agricultural Products. Toxins, 12(3), 158. https://doi.org/10.3390/toxins12030158