Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies
Abstract
:1. Introduction
2. Tumor-Promoting Activities of Tipα Family Members, HP-MP1, and Tipα
3. Secretion of Tipα from H. pylori
4. Cellular Response to rTipα
5. Crystal Structures of Rdel-Tipα and N-Terminal Truncated rTipα
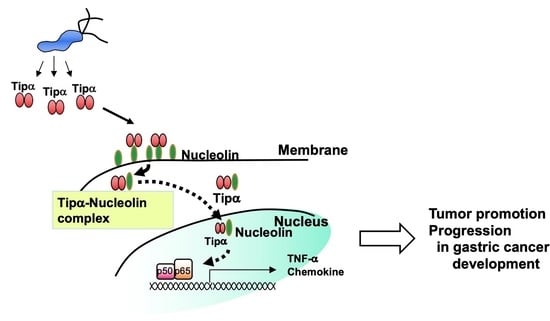
6. Nucleolin as a Cell-Surface Receptor for rTipα
7. Epithelial-Mesenchymal Transition (EMT) Induced by rTipα
8. Inhibition of Tipα-Associated Gastric Carcinogenesis by BTG2
9. New Strategies to Prevent and Treat Gastric Cancer
10. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, B.J.; Warren, J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984, 1, 1311–1315. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. The Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994; Volume 61. [Google Scholar]
- Tomb, J.F.; White, O.; Kerlavage, A.R.; Clayton, R.A.; Sutton, G.G.; Fleischmann, R.D.; Ketchum, K.A.; Klenk, H.P.; Gill, S.; Dougherty, B.A.; et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 1997, 388, 539–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honda, S.; Fujioka, T.; Tokieda, M.; Satoh, R.; Nishizono, A.; Nasu, M. Development of Helicobacter pylori-induced gastric carcinoma in Mongolian gerbils. Cancer Res. 1998, 58, 4255–4259. [Google Scholar]
- Watanabe, T.; Tada, M.; Nagai, H.; Sasaki, S.; Nakao, M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology 1998, 115, 642–648. [Google Scholar] [CrossRef]
- Shimizu, N.; Inada, K.; Nakanishi, H.; Tsukamoto, T.; Ikehara, Y.; Kaminishi, M.; Kuramoto, S.; Sugiyama, A.; Katsuyama, T.; Tatematsu, M. Helicobacter pylori infection enhances glandular stomach carcinogenesis in Mongolian gerbils treated with chemical carcinogens. Carcinogenesis 1999, 20, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Correa, P. Helicobacter pylori infection and gastric cancer. Cancer Epidemiol. Biomark. Prev. 2003, 12, 238s–241s. [Google Scholar]
- Kodaman, N.; Pazos, A.; Scheider, B.G.; Piazuelo, M.B.; Mera, R.; Sobota, R.S.; Sicinschi, L.A.; Shaffer, C.L.; Romero-Gallo, J.; de Sablet, T.; et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proc. Natl. Acad. Sci. USA 2014, 111, 1455–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montano, V.; Didelot, X.; Foll, M.; Linz, B.; Reinhardt, R.; Suerbaum, S.; Moodley, Y.; Jensen, J.D. Worldwide population structure, long-term demography, and local adaptation of Helicobacter pylori. Genetics 2015, 200, 947–963. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, T.; Fukuda, S.; Tanaka, M.; Mikami, T.; Saito, Y.; Munakata, A. High prevalence of the CagA-positive Helicobacter pylori strains in Japanese asymptomatic patients and gastric cancer patients. Scand. J. Gastroenterol. 1997, 32, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Lee, H.; Kim, M.; Fukumoto, M.; Sawada, S.; Jakate, S.; Gould, V.E. Ethnic difference of Helicobacter pylori gastritis: Korean and Japanese gastritis is characterized by male- and antrum-predominant acute foveolitis in comparison with American gastritis. World J. Gastroenterol. 2005, 11, 94–98. [Google Scholar] [CrossRef]
- Noto, J.M.; Peek, R.M.J. The Helicobacter pylori cag pathogenicity island. Methods Mol. Biol. 2012, 921, 41–50. [Google Scholar]
- Correa, P.; Piazuelo, M.B. Evolutionary history of the Helicobacter pylori genome: Implications for gastric carcinogenesis. Gut Liver 2012, 6, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Alm, R.A.; Ling, L.L.; Moir, D.T.; King, B.L.; Brown, E.D.; Doig, P.C.; Smith, D.R.; Noonan, B.; Guild, B.C.; deJonge, B.L.; et al. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter Pylori. Nature 1999, 397, 176–180. [Google Scholar] [CrossRef]
- Virchow, R. Reizung und Reizbarkeit. Arch. Pathol. Anat Physiol. Klin Z Med. 1858, 14, 1–63. [Google Scholar] [CrossRef]
- Van Durren, B.L. Tumor-promoting agents in two-stage carcinogenesis. Prog. Exp. Tumor Res. 1969, 11, 31–68. [Google Scholar]
- Komori, A.; Yatsunami, J.; Suganuma, M.; Okabe, S.; Abe, S.; Sakai, A.; Sakai, K.; Fujiki, H. Tumor necrosis factor acts as a tumor promoter in BALB/3T3 cell transformation. Cancer Res. 1993, 53, 1982–1985. [Google Scholar] [PubMed]
- Fujiki, H.; Sueoka, E.; Suganuma, M. Tumor promoters: From chemicals to inflammatory proteins. J. Cancer Res. Clin. Oncol. 2013, 139, 1603–1614. [Google Scholar] [CrossRef]
- Suganuma, M.; Okabe, S.; Marino, M.W.; Sakai, A.; Sueoka, E.; Fujiki, H. Essential role of tumor necrosis factor α (TNF-α) in tumor promotion revealed by TNF-α-deficient mice. Cancer Res. 1999, 59, 4516–4518. [Google Scholar] [PubMed]
- Yoshida, M.; Wakatsuki, Y.; Kobayashi, Y.; Itoh, T.; Murakami, K.; Mizoguchi, A.; Usui, T.; Chiba, T.; Kita, T. Cloning and characterization of a novel membrane-associated antigenic protein of Helicobacter Pylori. Infect. Immun. 1999, 67, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Suganuma, M.; Kurusu, M.; Okabe, S.; Sueoka, N.; Yoshida, M.; Wakatsuki, Y.; Fujiki, H. Helicobacter pylori membrane protein 1: A new carcinogenic factor of Helicobacter pylori. Cancer Res. 2001, 61, 6356–6359. [Google Scholar]
- Suganuma, M.; Kurusu, M.; Suzuki, K.; Nishizono, A.; Murakami, K.; Fujioka, T.; Fujiki, H. New tumor necrosis factor-α-inducing protein released from Helicobacter pylori for gastric cancer progression. J. Cancer Res. Clin. Oncol. 2005, 131, 305–313. [Google Scholar] [CrossRef]
- Normark, S.; Nilsson, C.; Normark, B.H.; Hornef, M.W. Persistent infection with Helicobacter pylori and the development of gastric cancer. Adv. Cancer Res. 2003, 90, 63–89. [Google Scholar]
- Amendola, C.R.; Mahaffey, J.P.; Parker, S.J.; Ahearn, I.M.; Chen, W.C.; Zhou, M.; Court, H.; Shi, J.; Mendoza, S.L.; Morten, M.J.; et al. KRAS4A directly regulates hexokinase 1. Nature 2019, 576, 482–486. [Google Scholar] [CrossRef]
- Suganuma, M.; Yamaguchi, K.; Ono, Y.; Matsumoto, H.; Hayashi, T.; Ogawa, T.; Imai, K.; Kuzuhara, T.; Nishizono, A.; Fujiki, H. TNF-α-inducing protein, a carcinogenic factor secreted from H. pylori, enters gastric cancer cells. Int. J. Cancer 2008, 123, 117–122. [Google Scholar] [CrossRef]
- Suganuma, M.; Kuzuhara, T.; Yamaguchi, K.; Fujiki, H. Carcinogenic role of tumor necrosis factor-α-inducing protein of Helicobacter pylori in human stomach. J. Biochem. Mol. Biol. 2006, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, H.; Tsurumura, T.; Utsunomiya, H.; Kise, D.; Kuzuhara, T.; Watanabe, T.; Fujiki, H.; Suganuma, M. Structural basis for the Helicobacter pylori-carcinogenic TNF-α-inducing protein. Biochem. Biophys. Res. Commun. 2009, 388, 193–198. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsuge, H.; Imagawa, T.; Kise, D.; Hirano, K.; Beppu, M.; Takahashi, A.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Nucleolin as cell surface receptor for tumor necrosis factor-α inducing protein: A carcinogenic factor of Helicobacter pylori. J. Cancer Res. Clin. Oncol. 2010, 136, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.B.; Sabo, E.; Meitner, P.A.; Kim, S.S.; Cho, Y.; Kim, H.K.; Tavares, R.; Moss, S.F. Global analysis of the human gastric epithelial transcriptome altered by Helicobacter pylori eradication in vivo. Gut 2006, 55, 1717–1724. [Google Scholar] [CrossRef] [Green Version]
- Kuzuhara, T.; Suganuma, M.; Kurusu, M.; Fujiki, H. Helicobacter pylori-secreting protein Tipα is a potent inducer of chemokine gene expressions in stomach cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 287–296. [Google Scholar] [CrossRef]
- Godlewska, R.; Pawlowski, M.; Dzwonek, A.; Mikula, M.; Ostrowski, J.; Drela, N.; Jagusztyn-Krynicka, E.K. Tip-α (hp0596 gene product) is a highly immunogenic Helicobacter pylori protein involved in colonization of mouse gastric mucosa. Curr. Microbiol. 2008, 56, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.L.; Hao, B.; Zhang, G.-X.; Shi, R.H.; Cheng, W.F. Helicobacter pylori tumor necrosis factor-α inducing protein promotes cytokine expression via nuclear factor-κB. World J. Gastroenterol. 2013, 19, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Bieger, B.; Essen, L.O.; Oesterhelt, D. Crystal structure of halophilic dodecin: A novel, dodecameric flavin binding protein from Halobacterium salinarum. Structure 2003, 11, 375–385. [Google Scholar] [CrossRef] [Green Version]
- Tosi, T.; Cioci, G.; Jouravleva, K.; Dian, C.; Terradot, L. Structures of the tumor necrosis factor α inducing protein Tipα: A novel virulence factor from Helicobacter pylori. FEBS Lett. 2009, 583, 1581–1585. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Yoon, H.J.; Yoon, J.Y.; Kim, H.S.; Lee, S.J.; Kim, K.H.; Kim, D.J.; Jang, S.; Han, B.G.; Lee, B.I.; et al. Crystal structure of the TNF-α-Inducing protein (Tipα) from Helicobacter pylori: Insights into its DNA-binding activity. J. Mol. Biol. 2009, 392, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Suganuma, M.; Oka, K.; Fujiki, H. DNA-binding activity of TNF-α-inducing protein from Helicobacter pylori. Biochem. Biophys. Res. Commun. 2007, 362, 805–810. [Google Scholar] [CrossRef]
- Gao, M.; Li, D.; Hu, Y.; Zhang, Y.; Zou, Q.; Wang, D.C. Crystal structure of TNF-α inducing protein from Helicobacter pylori in active form reveals the intrinsic molecular flexibility for unique DNA-binding. PLoS ONE 2012, 7, e41871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storck, S.; Shukla, M.; Dimitrov, S.; Bouvet, P. Functions of the histone chaperone nucleolin in diseases. Subcell. Biochem. 2007, 41, 125–144. [Google Scholar]
- Watanabe, T.; Hirano, K.; Takahashi, A.; Yamaguchi, K.; Beppu, M.; Fujiki, H.; Suganuma, M. Nucleolin on the cell surface as a new molecular target for gastric cancer treatment. Biol. Pharm. Bull. 2010, 33, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Takahashi, A.; Suzuki, K.; Kurusu-Kanno, M.; Yamaguchi, K.; Fujiki, H.; Suganuma, M. Epithelial-mesenchymal transition in human gastric cancer cell lines induced by TNF-α-inducing protein of Helicobacter pylori. Int. J. Cancer 2014, 134, 2373–2382. [Google Scholar] [CrossRef]
- Watanabe, T.; Kuramochi, H.; Takahashi, A.; Imai, K.; Katsuta, N.; Nakayama, T.; Fujiki, H.; Suganuma, M. Higher cell stiffness indicating lower metastatic potential in B16 melanoma cell variants and in (-)-epigallocatechin gallate-treated cells. J. Cancer Res. Clin. Oncol. 2012, 138, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Tang, N.; Wang, C.; Xiao, L.; Yu, M.; Zhao, L.; Cai, H.; Han, L.; Xie, C.; Zhang, Y. TNF-α-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Vallese, F.; Mishra, N.M.; Pagliari, M.; Berto, P.; Codolo, G.; de Bernard, M.; Zanotti, G. Helicobacter pylori antigenic Lpp20 is a structural homologue of Tipα and promotes epithelial-mesenchymal transition. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.W.; Varnum, B.C.; Herschman, H.R. Cloning of tetradecanoyl phorbol ester-induced ‘primary response’ sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene 1987, 1, 263–270. [Google Scholar]
- Fletcher, B.S.; Lim, R.W.; Varnum, B.C.; Kujubu, D.A.; Koski, R.A.; Herschman, H.R. Structure and expression of TIS21, a primary response gene induced by growth factors and tumor promoters. J. Biol. Chem. 1991, 266, 14511–14518. [Google Scholar] [CrossRef]
- Rouault, J.P.; Falette, N.; Guéhenneux, F.; Guillot, C.; Rimokh, R.; Wang, Q.; Berhet, C.; Moyret-Lalle, C.; Savatier, P.; Pain, B.; et al. Identification of BTG2, an antiproliferative p53-dependent component of the DNA damage cellular response pathway. Nat. Genet. 1996, 14, 482–486. [Google Scholar] [CrossRef]
- Park, T.J.; Kim, J.Y.; Oh, S.P.; Kang, S.Y.; Kim, B.W.; Wang, H.J.; Song, K.Y.; Kim, H.C.; Lim, I.K. TIS21 negatively regulates hepatocarcinogenesis by disruption of cyclin B1-Forkhead box M1 regulation loop. Hepatology 2008, 47, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.B.; Park, T.J.; Lim, I.K. B cell translocation gene 2 enhances susceptibility of HeLa cells to doxorubicin-induced oxidative damage. J. Biol. Chem. 2008, 283, 33110–33118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devanand, P.; Oya, Y.; Sundaramoorthy, S.; Song, K.Y.; Watanabe, T.; Kobayashi, Y.; Shimizu, Y.; Hong, S.A.; Suganuma, M.; Lim, I.K. Inhibition of TNF-α-interacting protein α (Tipα)-associated gastric carcinogenesis by BTG2/TIS21 via downregulating cytoplasmic nucleolin expression. Exp. Mol. Med. 2018, 23, e449. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer Working Group. Helicobacter pylori eradication as a strategy for preventing gastric cancer. In IARC Working Group Reports; IARC: Lyon, France, 2014; Volume 8. [Google Scholar]
- Inoue, K.; Shiota, S.; Yamada, K.; Gotoh, K.; Suganuma, M.; Fukioka, T.; Ahmed, K.; Iha, H.; Nishizono, A. Evaluation of a new tumor necrosis factor-α-inducing membrane protein of Helicobacter pylori as a prophylactic vaccine antigen. Helicobacter 2009, 14, 135–143. [Google Scholar] [CrossRef]
- Nisole, S.; Krust, B.; Callebaut, C.; Guichard, G.; Muller, S.; Briand, J.P.; Hovanessian, A.G. The anti-HIV pseudopeptide HB-19 forms a complex with the cell-surface-expressed nucleolin independent of heparan sulfate proteoglycans. J. Biol. Chem. 1999, 274, 27875–27884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafaie, E.; Saberi, S.; Esmaeili, M.; Karimi, Z.; Najafi, S.; Tashakoripoor, M.; Abdirad, A.; Hosseini, M.E.; Mohagheghi, M.A.; Khalaj, V.; et al. Multiplex serology of Helicobacter pylori antigens in detection of current infection and atrophyic gastritis—A simple and cost-efficient method. Microb. Pathog. 2018, 119, 137–144. [Google Scholar] [CrossRef]
- Krust, B.; El Khoury, D.; Nondier, I.; Soundaramourty, C.; Hovanessian, A.G. Targeting surface nucleolin with multivalent HB-19 and related nucant pseudopeptides results in distinct inhibitory mechanisms depending on the malignant tumor cell type. BMC Cancer 2011, 11, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiki, H.; Watanabe, T.; Suganuma, M. Cell-surface nucleolin acts as a central mediator for carcinogenic, anti-carcinogenic, and disease-related ligands. J. Cancer Res. Clin. Oncol. 2014, 140, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Hatakeyama, M. Structure and function of Helicobacetr pylori CagA, the first-identified bacterial protein involved in human cancer. Proc. Jpn. Acad. Ser. B 2017, 93, 196–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClain, M.S.; Beckett, A.C.; Cover, T.L. Helicobacter pylori vacuolating toxin and gastric cancer. Toxin 2017, 9, 316. [Google Scholar] [CrossRef] [Green Version]
- Maeda, S.; Ogura, K.; Yoshida, H.; Kanai, F.; Ikenoue, T.; Kato, N.; Shiratori, Y.; Omata, M. Major virulence factors, VacA and CagA, are commonly positive in Helicobacter pylori isolated in Japan. Gut 1998, 42, 338–343. [Google Scholar] [CrossRef] [Green Version]
- Mahamoudi, S.; Mancini, E.; Xu, L.; Moore, A.; Jahanbani, F.; Hebestreit, K.; Srinvasan, R.; Li, X.; Devarajan, K.; Prélot, L.; et al. Heterogeneity in old fibroblasts is linked to variability in reprogramming and wound healing. Nature 2019, 574, 553–558. [Google Scholar] [CrossRef]





| Bhas 42 Cells (with v-H-ras) | BALB/3T3 Cells | |||
|---|---|---|---|---|
| tnf-α Gene Expression | Tumorigenicity/ Transformation | tnf-α Gene Expression | Tumorigenicity/ Transformation | |
| Transfection | (Bhas/mp1) | (BALB/mp1) | ||
| hp-mp1 gene | High | High | Very low | No |
| (Bhas/ure) | (BALB/ure) | |||
| urease B gene | Low | Low | Very low | No |
| Treatment | ||||
| rTipα protein | High | High | Low | No |
| rdel-Tipα protein | Low | - | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suganuma, M.; Watanabe, T.; Sueoka, E.; Lim, I.K.; Fujiki, H. Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies. Toxins 2021, 13, 181. https://doi.org/10.3390/toxins13030181
Suganuma M, Watanabe T, Sueoka E, Lim IK, Fujiki H. Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies. Toxins. 2021; 13(3):181. https://doi.org/10.3390/toxins13030181
Chicago/Turabian StyleSuganuma, Masami, Tatsuro Watanabe, Eisaburo Sueoka, In Kyoung Lim, and Hirota Fujiki. 2021. "Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies" Toxins 13, no. 3: 181. https://doi.org/10.3390/toxins13030181
APA StyleSuganuma, M., Watanabe, T., Sueoka, E., Lim, I. K., & Fujiki, H. (2021). Role of TNF-α-Inducing Protein Secreted by Helicobacter pylori as a Tumor Promoter in Gastric Cancer and Emerging Preventive Strategies. Toxins, 13(3), 181. https://doi.org/10.3390/toxins13030181