Composition and Acute Inflammatory Response from Tetraponera rufonigra Venom on RAW 264.7 Macrophage Cells
Abstract
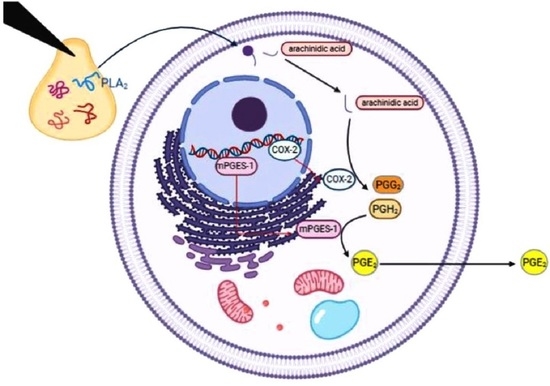
:1. Introduction
2. Results
2.1. Ant Characteristics
2.2. The Venom Protein Identification
2.3. Secreted Phospholipases A2 (sPLA2) Activity
2.4. RAW 264.7 Macrophage Cell Viability
2.5. Quantification of COX-2 in Venom-Treated Cells
2.6. Measurement of Prostaglandin E2 (PGE2)
2.7. The mRNA Expression of COX-2, mPGES-1, and cPLA2
3. Discussion
4. Materials and Methods
4.1. Ant Collection and Identification
4.2. Venom Extraction and Quantification of Venom Protein
4.3. Protein Electrophoresis
4.4. Protein Identification
4.5. Secreted Phospholipases A2 (sPLA2) Activity
4.6. Cell Culture
4.7. Cell Viability
4.8. Measurement of COX-2 and PGE2
4.9. Inflammatory-Released Molecule Gene Expression
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratnatilaka, G.A.; Herath, R.R.; Dias, R.K. Severe anaphylaxis following ant bites. Ceylon Med. J. 2011, 56, 34–35. [Google Scholar] [CrossRef] [Green Version]
- Potiwat, R.; Sitcharungsi, R. Ant allergens and hypersensitivity reactions in response to ant stings. Asian Pac. J. Allergy Immunol. 2015, 33, 267–275. [Google Scholar]
- Wanotayan, K.; Malainual, N.; Sassa-deepang, T.; Boonchoo, S.; Jirapongsananuruk, O.; Vichyanond, P. Anaphylaxis to venom of Tetraponera ruflonigra ant: A case report. J. Allergy Clin. Immunol. 2005, 115, S39. [Google Scholar] [CrossRef]
- Aili, S.R.; Touchard, A.; Escoubas, P.; Padula, M.P.; Orivel, J.; Dejean, A.; Nicholson, G.M. Diversity of peptide toxins from stinging ant venoms. Toxicon 2014, 92, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Touchard, A.; Aili, S.R.; Fox, E.G.; Escoubas, P.; Orivel, J.; Nicholson, G.M.; Dejean, A. The Biochemical Toxin Arsenal from Ant Venoms. Toxins 2016, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.O. Chemistry, pharmacology and chemical ecology of ant venoms. Venoms Hymenopt. 1986, 425–508. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.K.; Jeong, K.Y.; Lyu, D.P.; Lee, Y.W.; Sohn, J.H.; Lim, K.J.; Hong, C.S.; Park, J.W. Characterization of the major allergens of Pachycondyla chinensis in ant sting anaphylaxis patients. Clin. Exp. Allergy 2009, 39, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Barassé, V.; Touchard, A.; Téné, N.; Tindo, M.; Kenne, M.; Klopp, C.; Dejean, A.; Bonnafé, E.; Treilhou, M. The peptide venom composition of the fierce stinging ant Tetraponera aethiops (Formicidae: Pseudomyrmecinae). Toxins 2019, 11, 732. [Google Scholar] [CrossRef] [Green Version]
- Merlin, P.; Braekman, J.C.; Daloze, D.; Pasteels, J.M. Tetraponerines, toxic alkaloids in the venom of the Neo-Guinean pseudomyrmecine antTetraponera sp. J. Chem. Ecol. 1988, 14, 517–527. [Google Scholar] [CrossRef]
- Sousa, P.L.; Quinet, Y.P.; Brizeno, L.A.C.; Sampaio, T.L.; Torres, A.F.C.; Martins, A.M.C.; Assreuy, A.M.S. The acute inflammatory response induced in mice by the venom of the giant ant Dinoponera quadriceps involves macrophage and interleukin-1β. Toxicon 2016, 117, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pessoa, W.F.B.; Silva, L.C.C.; de Oliveira Dias, L.; Delabie, J.H.C.; Costa, H.; Romano, C.C. Analysis of protein composition and bioactivity of Neoponera villosa venom (Hymenoptera: Formicidae). Int. J. Mol. Sci. 2016, 17, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, B.; Tabarean, I.; Andrei, C.; Bartfai, T. Cytokines and fever. Front. Biosci. 2004, 9, 1433–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsson, K.; Kock, A.; Idborg, H.; Arsenian Henriksson, M.; Martinsson, T.; Johnsen, J.I.; Korotkova, M.; Kogner, P.; Jakobsson, P.J. COX/mPGES-1/PGE2 pathway depicts an inflammatory-dependent high-risk neuroblastoma subset. Proc. Natl. Acad. Sci. USA 2015, 112, 8070–8075. [Google Scholar] [CrossRef] [Green Version]
- Peavy, R.D.; Metcalfe, D.D. Understanding the mechanisms of anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 310–315. [Google Scholar] [CrossRef]
- Peebles, R.S., Jr. Prostaglandins in asthma and allergic diseases. Pharmacol. Ther. 2019, 193, 1–19. [Google Scholar] [CrossRef]
- Tuure, L.; Hämäläinen, M.; Whittle, B.J.; Moilanen, E. Microsomal Prostaglandin E Synthase-1 Expression in Inflammatory Conditions Is Downregulated by Dexamethasone: Seminal Role of the Regulatory Phosphatase MKP-1. Front. Pharmacol. 2017, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Sampey, A.V.; Monrad, S.; Crofford, L.J. Microsomal prostaglandin E synthase-1: The inducible synthase for prostaglandin E2. Arthritis Res. Ther. 2005, 7, 114–117. [Google Scholar] [CrossRef] [Green Version]
- Cologna, C.T.; Rodrigues, R.S.; Santos, J.; De Pauw, E.; Arantes, E.C.; Quinton, L. Peptidomic investigation of Neoponera villosa venom by high-resolution mass spectrometry: Seasonal and nesting habitat variations. J. Venom. Anim. Toxins Trop. Dis. 2018, 24, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Danneels, E.L.; Van Vaerenbergh, M.; Debyser, G.; Devreese, B.; De Graaf, D.C. Honeybee venom proteome profile of queens and winter bees as determined by a mass spectrometric approach. Toxins 2015, 7, 4468–4483. [Google Scholar] [CrossRef] [Green Version]
- Benmoussa, K.; Authier, H.; Prat, M.; AlaEddine, M.; Lefèvre, L.; Rahabi, M.C.; Bernad, J.; Aubouy, A.; Bonnafé, E.; Leprince, J.; et al. P17, an Original Host Defense Peptide from Ant Venom, Promotes Antifungal Activities of Macrophages through the Induction of C-Type Lectin Receptors Dependent on LTB4-Mediated PPARγ Activation. Front. Immunol. 2017, 8, 1650. [Google Scholar] [CrossRef]
- Perez-Riverol, A.; Lasa, A.M.; dos Santos-Pinto, J.R.A.; Palma, M.S. Insect venom phospholipases A1 and A2: Roles in the envenoming process and allergy. Insect Biochem. Mol. Biol. 2019, 105, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.F.; Huang, C.; Chong, C.M.; Leung, S.W.; Prieto-da-Silva, A.R.; Havt, A.; Quinet, Y.P.; Martins, A.M.; Lee, S.M.; Rádis-Baptista, G. Transcriptome analysis in venom gland of the predatory giant ant Dinoponera quadriceps: Insights into the polypeptide toxin arsenal of hymenopterans. PLoS ONE 2014, 9, e87556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, M.F.; Mota, C.M.; Miranda Vdos, S.; Cunha Ade, O.; Silva, M.C.; Naves, K.S.; de Oliveira, F.; Silva, D.A.; Mineo, T.W.; Santiago, F.M. Biological and Enzymatic Characterization of Proteases from Crude Venom of the Ant Odontomachus bauri. Toxins 2015, 7, 5114–5128. [Google Scholar] [CrossRef]
- Sabtu, F.S.; Ab Majid, A.H. Genetic variation and population structure of the arboreal bicolored ant Tetraponera rufonigra Jerdon from selected urban locations in eastern Penang Island, Malaysia. J. Asia Pac. Entomol. 2017, 20, 1350–1357. [Google Scholar] [CrossRef]
- Dhananjaya, B.L.; D’Souza, C.J. The pharmacological role of nucleotidases in snake venoms. Cell Biochem. Funct. Cell. Biochem. Modul. Act. Agents Dis. 2010, 28, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Duan, Z.; Di, Z.; He, Y.; Li, J.; Li, Z.; Xie, C.; Zeng, X.; Cao, Z.; Wu, Y. Proteomic analysis of the venom from the scorpion Mesobuthus martensii. J. Proteom. 2014, 106, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.P.; Games, P.D.; Azevedo, D.O.; Barros, E.; de Oliveira, L.L.; de Oliveira Ramos, H.J.; Baracat-Pereira, M.C.; Serrão, J.E. Proteomic analysis of the venom of the predatory ant Pachycondyla striata (Hymenoptera: Formicidae). Arch. Insect Biochem. Physiol. 2017, 96, e21424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, Z.-W.; Xiong, S.-J.; Xu, G.; Gan, S.-Y.; Chen, X.; Stanley, D.; Yan, Z.-C.; Ye, G.-Y.; Fang, Q. Protein discovery: Combined transcriptomic and proteomic analyses of venom from the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). Toxins 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Arvidson, R.; Kaiser, M.; Lee, S.S.; Urenda, J.-P.; Dail, C.; Mohammed, H.; Nolan, C.; Pan, S.; Stajich, J.E.; Libersat, F. Parasitoid jewel wasp mounts multipronged neurochemical attack to hijack a host brain. Mol. Cell. Proteom. 2019, 18, 99–114. [Google Scholar] [CrossRef] [Green Version]
- Palm, N.W.; Rosenstein, R.K.; Yu, S.; Schenten, D.D.; Florsheim, E.; Medzhitov, R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity 2013, 39, 976–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochoumian, L.; Joslyn, A. Wasp venom proteins: Phospholipase A1 and B. Arch. Biochem. Biophys. 1984, 230, 1–12. [Google Scholar]
- Schmidt, J.O.B.; Murray, S. The biochemical constituents of the venom of the harvester ant, Pogonomyrmex badius. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1978, 61, 239–247. [Google Scholar] [CrossRef]
- Chen, S.C.; Muller, M.; Zhou, J.Z.; Wright, L.C.; Sorrell, T.C. Phospholipase activity in Cryptococcus neoformans: A new virulence factor? Int. J. Infect. Dis. 1997, 175, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaako, K.; Waniek, A.; Parik, K.; Klimaviciusa, L.; Aonurm-Helm, A.; Noortoots, A.; Anier, K.; Van Elzen, R.; Gérard, M.; Lambeir, A.-M. Prolyl endopeptidase is involved in the degradation of neural cell adhesion molecules in vitro. J. Cell Sci. 2016, 129, 3792–3802. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Chen, L. An update of microsomal prostaglandin E synthase-1 and PGE2 receptors in cardiovascular health and diseases. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Ribardo, D.A.; Crowe, S.E.; Kuhl, K.R.; Peterson, J.W.; Chopra, A.K. Prostaglandin levels in stimulated macrophages are controlled by phospholipase A2-activating protein and by activation of phospholipase C and D. J. Biol. Chem. 2001, 276, 5467–5475. [Google Scholar] [CrossRef] [Green Version]
- Linkous, A.; Yazlovitskaya, E. Cytosolic phospholipase A2 as a mediator of disease pathogenesis. Cell. Microbiol. 2010, 12, 1369–1377. [Google Scholar] [CrossRef]
- Pearson, W.R. An introduction to sequence similarity (“homology”) searching. Curr. Protoc. Bioinform. 2013, 42, 3.1.1–3.1.8. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Wu, J.; Liang, Z.; Deng, F.; Xie, W.; Zhu, M.; Zhu, J.; Zhu, W.; Geng, S. Anti-inflammatory activity of magnesium isoglycyrrhizinate through inhibition of phospholipase A2/arachidonic acid pathway. Inflammation 2015, 38, 1639–1648. [Google Scholar] [CrossRef]
- Han, M.; Wen, J.-k.; Zheng, B.; Zhang, D.-Q. Acetylbritannilatone suppresses NO and PGE2 synthesis in RAW 264.7 macrophages through the inhibition of iNOS and COX-2 gene expression. Life Sci. 2004, 75, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Masuko-Hongo, K.; Berenbaum, F.; Humbert, L.; Salvat, C.; Goldring, M.B.; Thirion, S. Up-regulation of microsomal prostaglandin E synthase 1 in osteoarthritic human cartilage: Critical roles of the ERK-1/2 and p38 signaling pathways. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2004, 50, 2829–2838. [Google Scholar] [CrossRef] [PubMed]







| Band | Accession Code | Protein | Peptide Sequences | Query Cover | E-Value | % Identify |
|---|---|---|---|---|---|---|
| A | EFN75173.1 | Paramyosin, long form | LDLER, IISKLEAR, VQLEEESEAR | 100% | 6 × 10−4 | 87% |
| KYN11253.1 | Paramyosin, short form | IDLER, IISKLEAR | 100% | 0.41 | 100% | |
| B | XP_011143765.1 | protein 5NUC | IGVIGYLTPETK, EVEDIDLVIGGHTNTFLYR, KVYVVQAYAYTK | 100% | 4 × 10−10 | 53% |
| XP_011253192.1 | Protein 5NUC | SESPSTIFLNAGDTYQGTAWYNVYK, KVYVVQAYAYTK, GDIISVLPFGNVIVK | 100% | 2 × 10−10 | 100% | |
| XP_024877450.1 | protein 5NUC-like | KVYVVQAYAYTK, DDQVTRADVISVLPFGNVIVK | 100% | 4 × 10−6 | 82% | |
| XP_025991233.1 | Protein 5NUC | EVEDIDLVIGGHTNTFLYR, KVYVVQAYAYTK, ADIISVLPFGNVIVK | 100% | 3 × 10−9 | 57% | |
| C | XP_012521687.1 | PREDICTED:prolyl endopeptidase-like | FLDPFLDVVTK, FLNPFLDVVTK | 81% | 6.3 | 75% |
| XP_012146606.1 | PREDICTED: von Willebrand factor A domain-containing protein 8 isoform X1 | AVKIANTIAEIFK | 100% | 1.8 | 69% | |
| XP_018374341.1 | PREDICTED: transcriptional activator cubitus interruptus | SGGGGGGGLGSGGSIR | 87% | 0.03 | 93% | |
| EZA61259.1 | Aminopeptidase N | HKSLDDFSNGK | 72% | 4.5 | 88% | |
| OAD53823.1 | Aminopeptidase N | FLGIGTLSR | 72% | 4.5 | 88% | |
| XP_018301168.1 | PREDICTED: growth arrest and DNA damage-inducible proteins-interacting protein 1 | AQYEDLAKK | 88% | 1.5 | 88% | |
| XP_012219589.1 | PREDICTED: jmjC domain-containing protein 4 isoform X2 | WVYILDGATFEVLR | 100% | 0.24 | 73% | |
| XP_011145278.1 | ATP-binding cassette sub-family A member 3 | SGMDPEK + Oxidation(M) | 85% | 15 | 100% | |
| D | PRD20637.1 | Trypsin-3 | LGEDNINVVEGNEQFISASK, SIVHPSYNS NTLNNDIMLIK | 97% | 3 × 10−7 | 46% |
| ARK19907.1 | venom protein | SLDLDSIIAEVK, WELLQQVDTSTR | 87% | 0.024 | 59% | |
| E | XP_011139048.1 | protein jagged-1 | LLARPLARPLALHTR | 80% | 0.013 | 91% |
| F | KFM72666.1 | hypothetical protein X975_20223 | LGEDNINVVEGNEQFISASK, SSGTSYPDVLK | 83% | 5.2 | 42% |
| G | KMQ91113.1 | gag-pol polyprotein | SVGLDDSIR | 88% | 1.1 | 100% |
| XP_024886252.1 | transcription factor SPT20 homolog isoform X1 | SVGLVEAER | 88% | 12 | 88% | |
| XP_011262712.1 | phospholipase A2 | NDGLFTR | 100% | 16 | 86% |
| Group of Proteins | Type of Protein |
|---|---|
| 1. The venom protein | protein 5NUC prolyl endopeptidase-like aminopeptidase N trypsin-3 venom protein phospholipase A2 |
| 2. The transcription activator/regulation protein | transcriptional activator cubitus interruptus |
| 3. Cell cycle control protein | growth arrest and DNA damage-inducible proteins-interacting protein 1 |
| 4. Transporter protein | ATP-binding cassette sub-family A member 3 |
| 5. Structural protein | Paramyosin, long form Paramyosin short form |
| 6. Ligand protein | protein jagged-1 |
| 7. hypothetical protein | - |
| Target Genes | Sequences | Reference |
|---|---|---|
| cPLA2 | forward 5′–CAT TTA ACC TGC CAT ATC CCT-3′ Reverse- 5′–ATG GTT GGG CAA TCC TT-3′ | [40] |
| COX-2 | forward 5′ -GAA GTC TTT GGT CTG GTG CCT G-3′ Reverse 5′ -GTC TGC TGG TTT GGA ATA GTT GC-3′ | [41] |
| mPGES-1 | forward 5′- GGA TGC GCT GAA ACG TGG A- 3′ Reverse 5′- CAG GAA TGA GTA CAC GAA GCC - 3′ | [42] |
| GAPDH | Forward 5′-ACC ACA GTC CAT GCC ATC AC-3′ Reverse- 5′-TCC ACC ACC CTG TTG CTG TA-3′ | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naephrai, S.; Khacha-ananda, S.; Pitchakarn, P.; Jaikang, C. Composition and Acute Inflammatory Response from Tetraponera rufonigra Venom on RAW 264.7 Macrophage Cells. Toxins 2021, 13, 257. https://doi.org/10.3390/toxins13040257
Naephrai S, Khacha-ananda S, Pitchakarn P, Jaikang C. Composition and Acute Inflammatory Response from Tetraponera rufonigra Venom on RAW 264.7 Macrophage Cells. Toxins. 2021; 13(4):257. https://doi.org/10.3390/toxins13040257
Chicago/Turabian StyleNaephrai, Suwatjanee, Supakit Khacha-ananda, Pornsiri Pitchakarn, and Churdsak Jaikang. 2021. "Composition and Acute Inflammatory Response from Tetraponera rufonigra Venom on RAW 264.7 Macrophage Cells" Toxins 13, no. 4: 257. https://doi.org/10.3390/toxins13040257
APA StyleNaephrai, S., Khacha-ananda, S., Pitchakarn, P., & Jaikang, C. (2021). Composition and Acute Inflammatory Response from Tetraponera rufonigra Venom on RAW 264.7 Macrophage Cells. Toxins, 13(4), 257. https://doi.org/10.3390/toxins13040257