Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece
Abstract
:1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Parameters of Lake Vegoritis
2.2. Chlorophyll α
2.3. Phytoplankton
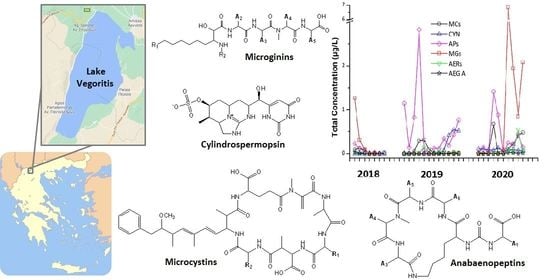
2.4. Occurrence of Cyanotoxins (CTs) in Lake Vegoritis
2.5. Detection, Identification and Occurrence of Cyanobacterial Peptides (CPs) in Lake Vegoritis
2.5.1. Chromatographic Separation and MS/MS Identification of CPs
2.5.2. Method Performance and Validation Results
2.5.3. Occurrence of CPs in Lake Vegoritis
3. Conclusions
4. Materials and Methods
4.1. Study Area Description and Sample Collection
4.2. Chemicals and Instrumentation
4.3. Physico-Chemical Parameters
4.4. Chlorophyll α Analysis
4.5. Microscopic Analysis
4.6. Analysis of CTs and CPs
4.6.1. Sample Preparation
4.6.2. LC-MS/MS Analysis
4.6.3. Validation of Methods for the Determination of CPs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meriluoto, J.; Spoof, L.; Codd, G.A. Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Wiley: Chichester, West Sussex, UK, 2017; pp. 1–548. [Google Scholar]
- Van Apeldoorn, M.E.; Van Egmond, H.P.; Speijers, G.J.A.; Bakker, G.J.I. Toxins of cyanobacteria. Mol. Nutr. Food Res. 2007, 51, 7–60. [Google Scholar] [CrossRef] [PubMed]
- Buratti, F.M.; Manganelli, M.; Vichi, S.; Stefanelli, M.; Scardala, S.; Testai, E.; Funari, E. Cyanotoxins: Producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 2017, 91, 1049–1130. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Azevedo, S.M.F.O.; An, J.S.; Molica, R.J.R.; Jochimsen, E.M.; Lau, S.; Rinehart, K.L.; Shaw, G.R.; Eaglesham, G.K. Human fatalities form cyanobacteria: Chemical and biological evidence for cyanotoxins. Environ. Health Perspect. 2001, 109, 663–668. [Google Scholar] [CrossRef]
- Jochimsen, E.M.; Carmichael, W.W.; An, J.; Cardo, D.M.; Cookson, S.T.; Holmes, C.E.M.; De Antunes, M.B.C.; De Melo Filho, D.A.; Lyra, T.M.; Barreto, V.S.T.; et al. Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. N. Engl. J. Med. 1998, 338, 873–878. [Google Scholar] [CrossRef]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Pflugmacher, S. Contribution of hot spring cyanobacteria to the mysterious deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Briand, J.F.; Jacquet, S.; Bernard, C.; Humbert, J.F. Health hazards for terrestrial vertebrates from toxic cyanobacteria in surface water ecosystems. Vet. Res. 2003, 34, 361–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, D.; Fang, D. Nodularins in poisoning. Clin. Chim. Acta 2013, 425, 18–29. [Google Scholar] [CrossRef]
- Carmichael, W.W. The Cyanotoxins. In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 27, pp. 211–256. [Google Scholar]
- Dawson, R.M. The toxicology of microcystins. Toxicon 1998, 36, 953–962. [Google Scholar] [CrossRef]
- De La Cruz, A.A.; Hiskia, A.; Kaloudis, T.; Chernoff, N.; Hill, D.; Antoniou, M.G.; He, X.; Loftin, K.; O’Shea, K.; Zhao, C.; et al. A review on cylindrospermopsin: The global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ. Sci. Process. Impacts 2013, 15, 1979–2003. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Kokociński, M. Cylindrospermopsin: Water-linked potential threat to human health in Europe. Environ. Toxicol. Pharmacol. 2012, 34, 651–660. [Google Scholar] [CrossRef]
- Osswald, J.; Rellán, S.; Gago, A.; Vasconcelos, V. Toxicology and detection methods of the alkaloid neurotoxin produced by cyanobacteria, anatoxin-a. Environ. Int. 2007, 33, 1070–1089. [Google Scholar] [CrossRef]
- Welker, M.; Von Döhren, H. Cyanobacterial peptides-Nature’s own combinatorial biosynthesis. FEMS Microbiol. Rev. 2006, 30, 530–563. [Google Scholar] [CrossRef] [Green Version]
- Okino, T.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Microginin, an angiotensin-converting enzyme inhibitor from the blue-green alga Microcystis aeruginosa. Tetrahedron Lett. 1993, 34, 501–504. [Google Scholar] [CrossRef]
- Janssen, E.M.L. Cyanobacterial peptides beyond microcystins–A review on co-occurrence, toxicity, and challenges for risk assessment. Water Res. 2019, 151, 488–499. [Google Scholar] [CrossRef]
- Ersmark, K.; Del Valle, J.R.; Hanessian, S. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. 2008, 47, 1202–1223. [Google Scholar] [CrossRef]
- Kohler, E.; Grundler, V.; Häussinger, D.; Kurmayer, R.; Gademann, K.; Pernthaler, J.; Blom, J.F. The toxicity and enzyme activity of a chlorine and sulfate containing aeruginosin isolated from a non-microcystin-producing Planktothrix strain. Harmful Algae 2014, 39, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Cegłowska, M.; Szubert, K.; Wieczerzak, E.; Kosakowska, A.; Mazur-Marzec, H. Eighteen New Aeruginosamide Variants Produced by the Baltic Cyanobacterium Limnoraphis CCNP1324. Mar. Drugs 2020, 18, 446. [Google Scholar] [CrossRef] [PubMed]
- Lawton, L.A.; Morris, L.A.; Jaspars, M. A bioactive modified peptide, aeruginosamide, isolated from the cyanobacterium Microcystis aeruginosa. J. Org. Chem. 1999, 64, 5329–5332. [Google Scholar] [CrossRef]
- Beversdorf, L.J.; Weirich, C.A.; Bartlett, S.L.; Miller, T.R. Variable cyanobacterial toxin and metabolite profiles across six eutrophic lakes of differing physiochemical characteristics. Toxins 2017, 9, 62. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, S.L.; Brunner, S.L.; Klump, J.V.; Houghton, E.M.; Miller, T.R. Spatial analysis of toxic or otherwise bioactive cyanobacterial peptides in Green Bay, Lake Michigan. J. Great Lakes Res. 2018, 44, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Usui, T.; Ueda, K.; Osada, H.; Kaya, K. Isolation of new protein phosphatase inhibitors from two cyanobacteria species, Planktothrix spp. J. Nat. Prod. 2001, 64, 1052–1055. [Google Scholar] [CrossRef]
- Sedmak, B.; Carmeli, S.; Eleršek, T. “Non-toxic” cyclic peptides induce lysis of cyanobacteria-An effective cell population density control mechanism in cyanobacterial blooms. Microb. Ecol. 2008, 56, 201–209. [Google Scholar] [CrossRef]
- Pelaez, M.; Antoniou, M.G.; He, X.; Dionysiou, D.D.; de la Cruz, A.A.; Tsimeli, K.; Triantis, T.; Hiskia, A.; Kaloudis, T.; Williams, C.; et al. Sources and Occurrence of Cyanotoxins Worldwide. In Xenobiotics in the Urban Water Cycle: Mass Flows, Environmental Processes, Mitigation and Treatment Strategies; Fatta-Kassinos, D., Bester, K., Kümmerer, K., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2010; pp. 101–127. [Google Scholar] [CrossRef]
- Balbus, J.M.; Boxall, A.B.A.; Fenske, R.A.; McKone, T.E.; Zeise, L. Implications of global climate change for the assessment and management of human health risks of chemicals in the natural environment. Environ. Toxicol. Chem. 2013, 32, 62–78. [Google Scholar] [CrossRef]
- Paerl, H.W.; Huisman, J. Climate change: A catalyst for global expansion of harmful cyanobacterial blooms. Environ. Microbiol. Rep. 2009, 1, 27–37. [Google Scholar] [CrossRef]
- Mantzouki, E.; Lürling, M.; Fastner, J.; de Senerpont Domis, L.; Wilk-Woźniak, E.; Koreivienė, J.; Seelen, L.; Teurlincx, S.; Verstijnen, Y.; Krztoń, W.; et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weirich, C.A.; Miller, T.R. Freshwater harmful algal blooms: Toxins and children’s health. Curr. Probl. Pediatric Adolesc. Health Care 2014, 44, 2–24. [Google Scholar] [CrossRef]
- Christophoridis, C.; Zervou, S.K.; Manolidi, K.; Katsiapi, M.; Moustaka-Gouni, M.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. Occurrence and diversity of cyanotoxins in Greek lakes. Sci. Rep. 2018, 8, 17877. [Google Scholar] [CrossRef] [Green Version]
- Zervou, S.K.; Gkelis, S.; Kaloudis, T.; Hiskia, A.; Mazur-Marzec, H. New microginins from cyanobacteria of Greek freshwaters. Chemosphere 2020, 248, 125961. [Google Scholar] [CrossRef]
- Zervou, S.K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazard. Mater. 2017, 323, 56–66. [Google Scholar] [CrossRef]
- Ministry of Environment and Energy. First Revision of the River Basin Management Plan of West Makedonia (EL09); Ministry of Environment and Energy: Athens, Greece, 2017; p. 296, Special Secretariat for Water. [Google Scholar]
- European Union. Directive 2006/7/EC of the European parliament and of the council of 15 February 2006 concerning the management of bathing water quality and repealing Directive 76/160/EEC. Off. J. Eur. Union 2006, 64, 37–51. [Google Scholar]
- Rastogi, R.P.; Sinha, R.P.; Incharoensakdi, A. The cyanotoxin-microcystins: Current overview. Rev. Environ. Sci. Bio/Technol. 2014, 13, 215–249. [Google Scholar] [CrossRef]
- Huang, I.S.; Zimba, P.V. Cyanobacterial bioactive metabolites—A review of their chemistry and biology. Harmful Algae 2019, 83, 42–94. [Google Scholar] [CrossRef] [PubMed]
- Cyanobacterial Toxins: Microcystins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- EN 16698:2015. Water Quality. Guidance on Quantitative and Qualitative Sampling of Phytoplankton from Inland Waters; Comité European de Normalization (CEN): Brussels, Belgium, 2015. [Google Scholar]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Spoof, L.; Catherine, A. Appendix 3: Tables of Microcystins and Nodularins. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; Wiley: Chichester, West Sussex, UK, 2017; pp. 526–537. [Google Scholar] [CrossRef]
- Kust, A.; Reháková, K.; Vrba, J.; Maicher, V.; Mareš, J.; Hrouzek, P.; Chiriac, M.-C.; Benedová, Z.; Tesarová, B.; Saurav, K. Insight into Unprecedented Diversity of Cyanopeptides in Eutrophic Ponds Using an MS/MS Networking Approach. Toxins 2020, 12, 561. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsuda, H.; Murakami, M.; Yamaguchi, K. Microginins 299-A and -B, leucine aminopeptidase inhibitors from the cyanobacterium Microcystis aeruginosa (NIES-299). Tetrahedron 1997, 53, 10281–10288. [Google Scholar] [CrossRef]
- Welker, M.; Maršálek, B.; Šejnohová, L.; von Döhren, H. Detection and identification of oligopeptides in Microcystis (cyanobacteria) colonies: Toward an understanding of metabolic diversity. Peptides 2006, 27, 2090–2103. [Google Scholar] [CrossRef] [PubMed]
- Zervou, S.K.; Kaloudis, T.; Hiskia, A.; Mazur-Marzec, H. Fragmentation mass spectra dataset of linear cyanopeptides-microginins. Data Brief 2020, 31, 105825. [Google Scholar] [CrossRef]
- Welker, M.; Brunke, M.; Preussel, K.; Lippert, I.; von Döhren, H. Diversity and distribution of Microcystis (cyanobacteria) oligopeptide chemotypes from natural communities studies by single-colony mass spectrometry. Microbiology 2004, 150, 1785–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Budnjo, A.; Jokela, J.; Haug, B.E.; Fewer, D.P.; Wahlsten, M.; Rouhiainen, L.; Permi, P.; Fossen, T.; Sivonen, K. Pseudoaeruginosins, Nonribosomal Peptides in Nodularia spumigena. ACS Chem. Biol. 2015, 10, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Erhard, M.; Von Döhren, H.; Jungblut, P.R. Rapid identification of the new anabaenopeptin G from Planktothrix agardhii HUB 011 using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 1999, 13, 337–343. [Google Scholar] [CrossRef]
- Häggqvist, K.; Toruńska-Sitarz, A.; Błaszczyk, A.; Mazur-Marzec, H.; Meriluoto, J. Morphologic, Phylogenetic and Chemical Characterization of a Brackish Colonial Picocyanobacterium (Coelosphaeriaceae) with Bioactive Properties. Toxins 2016, 8, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gkelis, S.; Lanaras, T.; Sivonen, K.; Taglialatela-Scafati, O. Cyanobacterial toxic and bioactive peptides in freshwater bodies of Greece: Concentrations, occurrence patterns, and implications for human health. Mar. Drugs 2015, 13, 6319–6335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, J.; Vasconcelos, V. Cyanobactins from Cyanobacteria: Current Genetic and Chemical State of Knowledge. Mar. Drugs 2015, 13, 6910–6946. [Google Scholar] [CrossRef] [Green Version]
- Österholm, J.; Popin, R.V.; Fewer, D.P.; Sivonen, K. Phylogenomic analysis of secondary metabolism in the toxic cyanobacterial genera Anabaena, Dolichospermum and aphanizomenon. Toxins 2020, 12, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anas, A.R.J.; Kisugi, T.; Umezawa, T.; Matsuda, F.; Campitelli, M.R.; Quinn, R.J.; Okino, T. Thrombin inhibitors from the freshwater cyanobacterium Anabaena compacta. J. Nat. Prod. 2012, 75, 1546–1552. [Google Scholar] [CrossRef]
- Harada, K.-i.; Fujii, K.; Shimada, T.; Suzuki, M.; Sano, H.; Adachi, K.; Carmichael, W.W. Two cyclic peptides, anabaenopeptins, a third group of bioactive compounds from the cyanobacteriumAnabaena flos-aquae NRC 525-17. Tetrahedron Lett. 1995, 36, 1511–1514. [Google Scholar] [CrossRef]
- Ferranti, P.; Fabbrocino, S.; Chiaravalle, E.; Bruno, M.; Basile, A.; Serpe, L.; Gallo, P. Profiling microcystin contamination in a water reservoir by MALDI-TOF and liquid chromatography coupled to Q/TOF tandem mass spectrometry. Food Res. Int. 2013, 54, 1321–1330. [Google Scholar] [CrossRef]
- Grabowska, M.; Kobos, J.; Toruńska-Sitarz, A.; Mazur-Marzec, H. Non-ribosomal peptides produced by Planktothrix agardhii from Siemianówka Dam Reservoir SDR (northeast Poland). Arch. Microbiol. 2014, 196, 697–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beversdorf, L.J.; Rude, K.; Weirich, C.A.; Bartlett, S.L.; Seaman, M.; Kozik, C.; Biese, P.; Gosz, T.; Suha, M.; Stempa, C.; et al. Analysis of cyanobacterial metabolites in surface and raw drinking waters reveals more than microcystin. Water Res. 2018, 140, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Roy-Lachapelle, A.; Vo Duy, S.; Munoz, G.; Dinh, Q.T.; Bahl, E.; Simon, D.F.; Sauvé, S. Analysis of multiclass cyanotoxins (microcystins, anabaenopeptins, cylindrospermopsin and anatoxins) in lake waters using on-line SPE liquid chromatography high-resolution Orbitrap mass spectrometry. Anal. Methods 2019, 11, 5289–5300. [Google Scholar] [CrossRef] [Green Version]
- Flores, C.; Caixach, J. High Levels of Anabaenopeptins Detected in a Cyanobacteria Bloom from N.E. Spanish Sau-Susqueda-El Pasteral Reservoirs System by LC–HRMS. Toxins 2020, 12, 541. [Google Scholar] [CrossRef]
- EN ISO 14911:1998. Water Quality. Determination of Dissolved Li+, Na+, NH4+, K+, Mn2+, Ca2+, Mg2+, Sr2+ and Ba2+ Using Ion Chromatography. Method for Water and Waste Water; Comité Europeen de Normalization (CEN): Brussels, Belgium, 1998. [Google Scholar]
- EN ISO 10304-1:2007. Water Quality–Determination of Dissolved Anions by Liquid Chromatography of Ions–Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate; Comité Europeen de Normalization (CEN): Brussels, Belgium, 2007. [Google Scholar]
- Rice, E.W.; Bridgewater, L.; American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- EN 15204:2006. Water Quality—Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique); Comité Europeen de Normalization (CEN): Brussels, Belgium, 2006. [Google Scholar]
- Utermohl, H. Zur Vervollkommung der quantitativen phytoplankton-methodik. Mitt. Int. Limnol. 1958, 9, 38. [Google Scholar]
- EN16695:2015. Water Quality–Guidance on the Estimation of Phytoplankton Biovolume; Comité Europeen de Normalization (CEN): Brussels, Belgium, 2015. [Google Scholar]
- Lombardo, M.; Pinto, F.C.R.; Vieira, J.M.S.; Honda, R.Y.; Pimenta, A.M.C.; Bemquerer, M.P.; Carvalho, L.R.; Kiyota, S. Isolation and structural characterization of microcystin-LR and three minor oligopeptides simultaneously produced by Radiocystis feernandoi (Chroococcales, Cyanobacteriae): A Brazilian toxic cyanobacterium. Toxicon 2006, 47, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Natumi, R.; Janssen, E.M.L. Cyanopeptide Co-Production Dynamics beyond Mirocystins and Effects of Growth Stages and Nutrient Availability. Environ. Sci. Technol. 2020, 54, 6063–6072. [Google Scholar] [CrossRef] [PubMed]








| CTs | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYN | dm MC-RR | MC-RR | MC-YR | MC-HtyR | dm MC-LR | MC-LR | MC-HilR | |||||||||
| % Presence | 71 | 12 | 50 | 24 | 7 | 17 | 79 | 5 | ||||||||
| CPs | ||||||||||||||||
| AP B | AP F | Osc Y | MG FR1 | MG FR3 | MG T1 | MG T2 | AER 602/K139 | AER 298A | AEG A | |||||||
| % Presence | 100 | 98 | 68 | 27 | 27 | 29 | 29 | 45 | 9 | 23 | ||||||
| Cyanopeptide | tR (min) | Precursor Ion | Product Ions | Collision Energy (eV) | Product Ion Assignment | Ref. |
|---|---|---|---|---|---|---|
| MG FR1 | 18.9 | 728.0 [M+H]+ | 100.0 | 40 | MeLeu immonium ion | [46] |
| 128.2 Q | 40 | Ahda fragment (C8H18N) | [45] | |||
| 384.2 | 40 | [M + H-Tyr-Tyr]+ | [46] | |||
| MG FR3 | 15.6 | 728.0 [Μ+H]+ | 128.2 | 40 | Ahda fragment (C8H18N) | [45] |
| 233.0 Q | 40 | [Pro-Tyr-CO + H]+ | [45] | |||
| 442.0 | 40 | [Pro-Tyr-Tyr + H]+ | [46] | |||
| MG T1 | 15.5 | 732.0 [Μ+H]+ | 162.1 | 40 | Cl-Ahda fragment (C8H17NCl) | [44] |
| 233.0 Q | 40 | [Pro-Tyr-CO + H]+ | [45] | |||
| 442.2 | 40 | [Pro-Tyr-Tyr + H]+ | [46] | |||
| MG T2 | 15.7 | 698.0 [M+H]+ | 128.2 | 40 | Ahda fragment (C8H18N) | [45] |
| 233.0 Q | 40 | [Pro-Tyr-CO + H]+ | [45] | |||
| 442.2 | 40 | [Pro-Tyr-Tyr + H]+ | [46] | |||
| AER 602/Κ139 | 13.8 | 603.2 [Μ+H]+ | 122.0 | 40 | [Choi immonium-H2O]+ | [47] |
| 140.0 | 40 | Choi immonium ion | [47] | |||
| 221.2 Q | 40 | Leu-Choi fragment | [45] | |||
| AER 298A | 13.6 | 605.3 [Μ+H]+ | 122.0 | 40 | [Choi immonium-H2O]+ | [47] |
| 140.0 | 40 | Choi immonium ion | [47] | |||
| 311.0 Q | 40 | [Choi-Argininol-NH2 + H]+ | [48] | |||
| AEG A | 24.6 | 561.4 [Μ+H]+ | 86.0 | 40 | [PreNH3]+ | This study |
| 112.0 | 40 | TzlCO | [20] | |||
| 154.2 Q | 40 | [(Pre)2NH2]+ | This study | |||
| AP B | 14.8 | 837.4 [Μ+H]+ | 84.0 | 40 | Lys immonium ion | [49] |
| 201.1 Q | 40 | CO-Arg (side chain) | [49] | |||
| 637.3 | 40 | [Lys-Phe-MeAla-HTyr-Val + 2H]+ | [49] | |||
| AP F | 15.2 | 851.3 [Μ+H]+ | 84.0 | 40 | Lys immonium ion | [49] |
| 201.0 Q | 40 | CO-Arg (side chain) | [49] | |||
| 651.4 | 40 | [Lys-Phe-MeAla-HTyr-Ile + 2H]+ | [49] | |||
| OSC Y | 19.9 | 858.4 [Μ+H]+ | 84.0 | 40 | Lys immonium ion | [50] |
| 405.0 Q | 40 | [M + H-Tyr-(Htyr-Ile)]+ | [50] | |||
| 681.4 | 40 | [M + H-Htyr]+ | [50] |
| MG FR1 | MG FR3 | MG T1 | MG T2 | AER 602/K139 | AER 298A | AEG A | AP B | AP F | Osc Y | |
|---|---|---|---|---|---|---|---|---|---|---|
| Extracellular Recovery (%RSD, n = 3) | 103.7% (9.5) | 79.7% (9.2) | 86.5% (6.7) | 77.0% (6.3) | 163.5% (7.4) | 129.2% (8.8) | 17.1% (25.2) | 102.7 (8.6) | 108.6% (6.5) | 95.8% (6.5) |
| Intracellular Recovery (%RSD, n = 3) | 74.0% (16.9) | 75.5% (2.2) | 75.1% (9.9) | 75.6% (13.8) | 88.8% (5.6) | 98.3% (13.7) | 7.5% (28.4) | 87.2% (3.3) | 96.5% (9.1) | 73.4% (10.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zervou, S.-K.; Moschandreou, K.; Paraskevopoulou, A.; Christophoridis, C.; Grigoriadou, E.; Kaloudis, T.; Triantis, T.M.; Tsiaoussi, V.; Hiskia, A. Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece. Toxins 2021, 13, 394. https://doi.org/10.3390/toxins13060394
Zervou S-K, Moschandreou K, Paraskevopoulou A, Christophoridis C, Grigoriadou E, Kaloudis T, Triantis TM, Tsiaoussi V, Hiskia A. Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece. Toxins. 2021; 13(6):394. https://doi.org/10.3390/toxins13060394
Chicago/Turabian StyleZervou, Sevasti-Kiriaki, Kimon Moschandreou, Aikaterina Paraskevopoulou, Christophoros Christophoridis, Elpida Grigoriadou, Triantafyllos Kaloudis, Theodoros M. Triantis, Vasiliki Tsiaoussi, and Anastasia Hiskia. 2021. "Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece" Toxins 13, no. 6: 394. https://doi.org/10.3390/toxins13060394
APA StyleZervou, S. -K., Moschandreou, K., Paraskevopoulou, A., Christophoridis, C., Grigoriadou, E., Kaloudis, T., Triantis, T. M., Tsiaoussi, V., & Hiskia, A. (2021). Cyanobacterial Toxins and Peptides in Lake Vegoritis, Greece. Toxins, 13(6), 394. https://doi.org/10.3390/toxins13060394