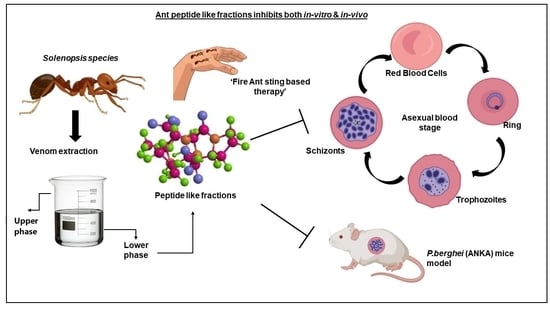
Studying the Rationale of Fire Ant Sting Therapy Usage by the Tribal Natives of Bastar Revealed Ant Venom-Derived Peptides with Promising Anti-Malarial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ant Collection and Venom Extraction
2.2. Protein Quantification by BCA Method
2.3. Extraction and Analysis of Venom Fractions by HPLC
2.4. Analysis of Venom-Derived Peptide Fractions in LC/MS
2.5. Cytotoxic Assay
2.6. Hemolytic Activity
2.7. In Vitro Antiplasmodial Activity
SYBR Green Assay
2.8. Animal Handling and Parasite Inoculation
2.9. In Vivo Anti-Malarial Activities
2.10. Statistical Analysis
3. Results
3.1. Quantification of Peptide-like Fractions by BCA Method
3.2. Effect of the Ant Venom Peptide Fraction against In Vitro Growth of Plasmodium falciparum
3.3. In Vivo Anti-Plasmodial Activity of the Peptide Fraction
3.4. Analysis of Venom Protein Fraction Using HPLC and LC/MS
- Database search: Genetic information of Solenopsis species implemented by using NCBI database (http://www.ncbi.nlm.-nih.gov/, accessed on 20 October 2022) and GenBank assembly accession: GCA_000188075.2(https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgiid=13686, accessed on 20 October 2022).
- Strain information: Laboratory strains Pf 3D7 (chloroquine-sensitive cell line) were obtained from the Malaria Parasite Bank at Malaria Research and Reference Reagent Resource Centre (MR4), Manassas, VA, USA.
- Both the databases accessed by 20 October 2022.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Global. World Malaria Report 2021; WHO Regional Office for Africa: Brazzaville, Congo, 2021. [Google Scholar]
- Ryan, E.T.; Solomon, T.; Endy, T.P. (Eds.) Hunter’s Tropical Medicine and Emerging Infectious Diseases. Hunter’s Tropical Medicine and Emerging Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Tavares, J.; Formaglio, P.; Thiberge, S.; Mordelet, E.; Van Rooijen, N.; Medvinsky, A.; Ménard, R.; Amino, R. Role of host cell traversal by the malaria sporozoite during liver infection. J. Exp. Med. 2013, 210, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mota, M.M.; Pradel, G.; Vanderberg, J.P.; Hafalla, J.C.R.; Frevert, U.; Nussenzweig, R.S.; Nussenzweig, V.; Rodriguez, A. Migration of Plasmodium sporozoites through cells before infection. Science 2001, 291, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hott, A.; Casandra, D.; Sparks, K.N.; Morton, L.C.; Castanares, G.G.; Rutter, A.; Kyle, D.E. Artemisinin-resistant Plasmodium falciparum parasites exhibit altered patterns of development in infected erythrocytes. Antimicrob. Agents Chemother. 2015, 59, 3156–3167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, N.J. Antimalarial drug resistance. J. Clin. Investig. 2004, 113, 1084–1092. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Billingham, M.E.; Morley, J.; Hanson, J.M.; Shipolini, R.A.; Vernon, C.A. Letter: An anti-inflammatory peptide from bee venom. Nature 1973, 245, 163–164. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Lee, H.J.; Han, H.J.; Mar, W.C.; Kang, S.K.; Yoon, O.B.; Alvin, J.B.; Lee, J.H. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci. 2002, 71, 191–204. [Google Scholar] [CrossRef]
- Zainal Abidin, S.A.; Lee, Y.Q.; Othman, I.; Naidu, R. Malaysian cobra venom: A potential source of anti-cancer therapeutic agents. Toxins 2019, 11, 75. [Google Scholar] [CrossRef]
- Alves, R.R.; Oliveira, T.P.; Rosa, I.L.; Cunningham, A.B. Marine invertebrates in traditional medicines. In Animals in Traditional Folk Medicine; Springer: Berlin/Heidelberg, Germany, 2013; pp. 263–287. [Google Scholar]
- Meyer-Rochow, V.B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: A comparative survey and review. J. Ethnobiol. Ethnomedicine 2017, 13, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Baek, Y.H.; Huh, J.E.; Lee, J.D.; Choi, D.Y.; Park, D.S. Antinociceptive effect and the mechanism of bee venom acupuncture (Apipuncture) on inflammatory pain in the rat model of collagen-induced arthritis: Mediation by α2-Adrenoceptors. Brain Res. 2006, 1073–1074, 305–310. [Google Scholar] [CrossRef]
- Choi, S.J.; Parent, R.; Guillaume, C.; Deregnaucourt, C.; Delarbre, C.; Ojcius, D.M.; Montagne, J.J.; Célérier, M.L.; Phelipot, A.; Amiche, M.; et al. Isolation and characterization of Psalmopeotoxin I and II: Two novel antimalarial peptides from the venom of the tarantula Psalmopoeus cambridgei. FEBS Lett. 2004, 572, 109–117. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.T.; Garcia Soares, A.; Stockand, J.D. Snake venoms in drug discovery: Valuable therapeutic tools for life saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.J.; Devine, D.A.; Bibby, M.C. Preliminary experimental anticancer activity of cecropins. Pept. Res. 1994, 7, 265–269. [Google Scholar] [PubMed]
- Conde, R.; Zamudio, F.Z.; Rodríguez, M.H.; Possani, L.D. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 2000, 471, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Jaimes, L.; Rodriguez, M.C.; Argotte-Ramos, R.; Juárez-González, V.R.; Pastor, N.; Cesa-Luna, C.; Possani, L.D.; Quintero-Hernández, V. Recombinant C-Terminal Domains from Scorpine-like Peptides Inhibit the Plasmodium berghei Ookinete Development In Vitro. Int. J. Pept. Res. Ther. 2020, 27, 817–829. [Google Scholar] [CrossRef]
- Santos, P.P.; Pereira, G.R.; Barros, E.; Ramos, H.J.O.; Oliveira, L.L.; Serrão, J.E. Antibacterial activity of the venom of the Ponerine ant Pachycondyla striata (Formicidae: Ponerinae). Int. J. Trop. Insect Sci. 2020, 40, 393–402. [Google Scholar] [CrossRef]
- Brady, S.G.; Larkin, L.; Danforth, B.N. Bees, Ants, and Stinging Wasps (Aculeata). In The Timetree of Life; Cornell University: New York, NY, USA, 2009. [Google Scholar]
- Orivel, J.; Redeker, V.; Le Caer, J.P.; Krier, F.; Revol-Junelles, A.M.; Longeon, A.; Chaffotte, A.; Dejean, A.; Rossier, J. Ponericins, New Antibacterial and Insecticidal Peptides from the Venom of the Ant Pachycondyla goeldii. J. Biol. Chem. 2001, 276, 17823–17829. [Google Scholar] [CrossRef] [Green Version]
- Barassé, V.; Touchard, A.; Téné, N.; Tindo, M.; Kenne, M.; Klopp, C.; Dejean, A.; Bonnafé, E.; Treilhou, M. The peptide venom composition of the fierce stinging ant tetraponera aethiops (formicidae: Pseudomyrmecinae). Toxins 2019, 11, 732. [Google Scholar] [CrossRef]
- Heep, J.; Skaljac, M.; Grotmann, J.; Kessel, T.; Seip, M.; Schmidtberg, H.; Vilcinskas, A. Identification and functional characterization of a novel insecticidal decapeptide from the myrmicine ant manica rubida. Toxins 2019, 11, 562. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, D.R. Venoms of the Hymenoptera: Biochemical, Pharmacological and Behavioural Aspects. Tom Piek. Q. Rev. Biol. 1987, 62, 331–332. [Google Scholar] [CrossRef]
- Schmidt, J.O. Biochemistry of insect venoms. Annu. Rev. Entomol. 1982, 27, 339–368. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P. Bugs as drugs, part 1: Insects. The “new” alternative medicine for the 21st century? Altern. Med. Rev. 2010, 15, 124–135. [Google Scholar] [PubMed]
- Kou, J.; Ni, Y.; Li, N.; Wang, J.; Liu, L.; Jiang, Z.H. Analgesic and anti-inflammatory activities of total extract and individual fractions of Chinese medicinal ants Polyrhachis lamellidens. Biol. Pharm. Bull. 2005, 28, 176–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.F.; Quinet, Y.P.; Havt, A.; Rádis-Baptista, G.; Martins, A.M. Molecular Pharmacology and Toxynology of Venom from Ants. An Integrated View of the Molecular Recognition and Toxinology—From Analytical Procedures to Biomedical Applications; IntechOpen: Rijeka, Croatia, 2013; pp. 207–222. [Google Scholar]
- Banks, W.A.; Lofgren, C.S.; Jouvenaz, D.P.; Stringer, C.E.; Bishop, P.M.; Williams, D.F.; Wojcik, D.P.; Glancey, B.M. Techniques for collecting, rearing, and handling imported fire ants. In Invasive Species Compendium; CABI: Wallingford, UK; Gulfport, MS, USA, 1981. [Google Scholar]
- Gonçalves Paterson Fox, E.; Russ Solis, D.; Delazari dos Santos, L.; Aparecido dos Santos Pinto, J.R.; Ribeiro da Silva Menegasso, A.; Cardoso Maciel Costa Silva, R.; Sergio Palma, M.; Correa Bueno, O.; de Alcântara Machado, E. A simple, rapid method for the extraction of whole fire ant venom (Insecta: Formicidae: Solenopsis). Toxicon 2013, 65, 5–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Fadamiro, H.Y. Re-investigation of venom chemistry of Solenopsis fire ants. I. Identification of novel alkaloids in S. richteri. Toxicon 2009, 53, 469–478. [Google Scholar] [CrossRef]
- Siddiqui, F.A.; Dhawan, S.; Singh, S.; Singh, B.; Gupta, P.; Pandey, A.; Mohmmed, A.; Gaur, D.; Chitnis, C.E. A thrombospondin structural repeat containing rhoptry protein from P lasmodium falciparum mediates erythrocyte invasion. Cell. Microbiol. 2013, 15, 1341–1356. [Google Scholar] [CrossRef]
- Kumari, G.; Rex, D.A.; Goswami, S.; Mukherjee, S.; Biswas, S.; Maurya, P.; Jain, R.; Garg, S.; Prasad, T.S.; Pati, S.; et al. Dynamic Palmitoylation of Red Cell Membrane Proteins Governs Susceptibility to Invasion by the Malaria Parasite, Plasmodium falciparum. ACS Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Kannan, D.; Yadav, N.; Ahmad, S.; Namdev, P.; Bhattacharjee, S.; Lochab, B.; Singh, S. Pre-clinical study of iron oxide nanoparticles fortified artesunate for efficient targeting of malarial parasite. eBioMedicine 2019, 45, 261–277. [Google Scholar] [CrossRef]
- Li, L.; Huang, J.; Lin, Y. Snake venoms in cancer therapy: Past, present and future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, B. Animal venoms have potential to treat cancer. Curr. Top. Med. Chem. 2019, 18, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, F.; Li, F.; Li, Z.; Lang, Y.; Shen, B.; Wu, Y.; Li, W.; Harrison, P.L.; Strong, P.N.; et al. Therapeutic potential of a scorpion venom-derived antimicrobial peptide and its homologs against antibiotic-resistant Gram-positive bacteria. Front. Microbiol. 2018, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Ju, W.; Chen, J.; Yuan, Y.; Zhang, C.; Liu, F.; Zhang, F. Retracted Article: Structural characterization and anti-inflammatory potency of Mesobuthus martensii Karsch oligopeptides in lipopolysaccharide (LPS)-induced RAW264. 7 macrophages. RSC Adv. 2019, 9, 24822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.M.; Fajloun, Z. Bee venom: Overview of main compounds and bioactivities for therapeutic interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- Mingomataj, E.C.; Bakiri, A.H. 1 Episodic Hemorrhage During Honeybee Venom Anaphylaxis: Potential Mechanisms. Investig. Allergy Clin. Immunol. 2012, 22, 237. [Google Scholar]
- Al-Tamimi, J.; Semlali, A.; Hassan, I.; Ebaid, H.; Alhazza, I.M.; Mehdi, S.H.; Al-Khalifa, M.; Alanazi, M.S. Samsum ant venom exerts anticancer activity through immunomodulation in vitro and in vivo. Cancer Biother. Radiopharm. 2018, 33, 65–73. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, J.; Sah, R.K.; Mohaideen. S, N.M.; Ahmad, S.; Pati, S.; Singh, S. Studying the Rationale of Fire Ant Sting Therapy Usage by the Tribal Natives of Bastar Revealed Ant Venom-Derived Peptides with Promising Anti-Malarial Activity. Toxins 2022, 14, 789. https://doi.org/10.3390/toxins14110789
Kumari J, Sah RK, Mohaideen. S NM, Ahmad S, Pati S, Singh S. Studying the Rationale of Fire Ant Sting Therapy Usage by the Tribal Natives of Bastar Revealed Ant Venom-Derived Peptides with Promising Anti-Malarial Activity. Toxins. 2022; 14(11):789. https://doi.org/10.3390/toxins14110789
Chicago/Turabian StyleKumari, Jyoti, Raj Kumar Sah, Nazar Mohamed Mohaideen. S, Shakeel Ahmad, Soumya Pati, and Shailja Singh. 2022. "Studying the Rationale of Fire Ant Sting Therapy Usage by the Tribal Natives of Bastar Revealed Ant Venom-Derived Peptides with Promising Anti-Malarial Activity" Toxins 14, no. 11: 789. https://doi.org/10.3390/toxins14110789
APA StyleKumari, J., Sah, R. K., Mohaideen. S, N. M., Ahmad, S., Pati, S., & Singh, S. (2022). Studying the Rationale of Fire Ant Sting Therapy Usage by the Tribal Natives of Bastar Revealed Ant Venom-Derived Peptides with Promising Anti-Malarial Activity. Toxins, 14(11), 789. https://doi.org/10.3390/toxins14110789