Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development and Characterization of Alginate–PVA–L. plantarum Complexes
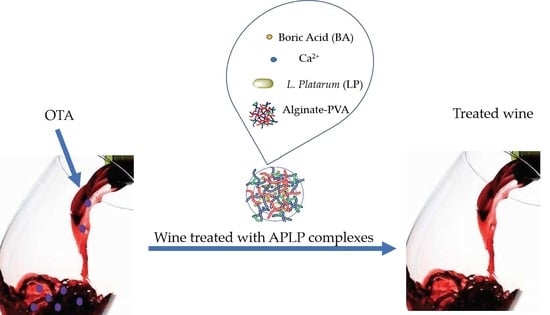
2.1.1. Development of Alginate–PVA–L. plantarum Complexes
2.1.2. Characterization of Alginate–PVA–L. plantarum Complexes
2.2. OTA Removal from Red Wines for APLP Complexes
2.2.1. Study of Effects of Complex Concentration on OTA and Phenol Removal
2.2.2. Optimization of OTA and Phenol Removal by APLP Complexes
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Medium
4.2. Bacterial Strain and Culture Condition
4.3. Immobilization of L. plantarum in Alginate-PVA and Bacterial Delivery Assay
4.4. Complex Characterization for Thermal Analysis by Thermogravimetry (TGA)
4.5. OTA Removal from Red Wines by AP and APLP Complexes
4.6. Analysis of OTA
4.6.1. OTA Extraction and Purification from Red Wine
4.6.2. Analysis of OTA by HPLC-FLD
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amezqueta, S.; Schorr-Galindo, S.; Murillo-Arbizu, M.; Gonzalez-Peñas, E.; De Cerain, A.L.; Guiraud, J. OTA-producing fungi in foodstuffs: A review. Food Control 2012, 26, 259–268. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Villeda, B.; Lobos, O.; Aguilar-Zuniga, K.; Carrasco-Sánchez, V. Ochratoxins in wines: A review of their occurrence in the last decade, toxicity, and exposure risk in humans. Toxins 2021, 13, 478. [Google Scholar] [CrossRef] [PubMed]
- Var, I.; Erginkaya, Z.; Kabak, B. Reduction of ochratoxin A levels in white wine by yeast treatments. J. Inst. Brew. 2009, 115, 30–34. [Google Scholar] [CrossRef]
- Zimmerli, B.; Dick, R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996, 13, 655–668. [Google Scholar] [CrossRef]
- Lasram, S.; Mani, A.; Zaied, C.; Chebil, S.; Abid, S.; Bacha, H.; Mliki, A.; Ghorbel, A. Evolution of ochratoxin A content during red and rose vinification. J. Sci. Food Agric. 2008, 88, 1696–1703. [Google Scholar] [CrossRef]
- Abrunhosa, L.; Fernandes, A.; Venâncio, A. Ochratoxin Aremoval during the main steps of wine making. In Proceedings of the 7 Encontro de Qu’ımica dos Alimentos, Viseu, Portugal, 13–16 April 2005; ESAV-IPV/SPQ: Viseu, Portugal, 2005. [Google Scholar]
- Dachery, B.; Veras, F.F.; Dal Magro, L.; Manfroi, V.; Welke, J.E. Exposure risk assessment to ochratoxin A through consumption of juice and wine considering the effect of steam extraction time and vinification stages. Food Chem. Toxicol. 2017, 109, 237–244. [Google Scholar] [CrossRef]
- Malir, F.; Louda, M.; Ostry, V.; Toman, J.; Ali, N.; Grosse, Y.; Malirova, E.; Pacovsky, J.; Pickova, D.; Brodak, M. Analyses of biomarkers of exposure to nephrotoxic mycotoxins in a cohort of patients with renal tumours. Mycotoxin Res. 2019, 35, 391–403. [Google Scholar] [CrossRef]
- Castegnaro, M.; Canadas, D.; Vrabcheva, T.; Petkova-Bocharova, T.; Chernozemsky, I.N.; Pfohl-Leszkowicz, A. Balkan endemic nephropathy: Role of ochratoxins A through biomarkers. Mol. Nutr. Food Res. 2006, 50, 519–529. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 123/2005 of 26 January 2005 amending regulation (EC) No 466/2001 as regards ochratoxin A. Off. J. Eur. Union 2005, 25, 3–5. [Google Scholar]
- European Commission. Commission Regulation EC No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R0401&from=ES (accessed on 16 June 2021).
- Solfrizzo, M.D.; Avantaggiato, G.; Panzarini, G.; Visconti, A. Removal of ochratoxin A from contaminated red wines by repassage over grape pomaces. J. Agric. Food Chem. 2010, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Castellari, M.; Versari, A.; Fabiani, A.; Parpinello, G.P.; Galassi, S. Removal of ochratoxin A in red wines by means of adsorption treatments with commercial fining agents. J. Agric. Food Chem. 2001, 49, 3917–3921. [Google Scholar] [CrossRef] [PubMed]
- Quintela, S.; Villarán, M.C.; López de Armentia, I.; Elejalde, E. Ochratoxin A removal in red wine by several oenological fining agents: Bentonite, egg albumin, allergen free adsorbents, chitin and chitosan. Food Addit. Contam. 2012, 29, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Nowak, A.; Czyzowska, A. Removal of ochratoxin A by wine Saccharomyces cerevisiae strains. Eur. Food Res. Technol. 2013, 236, 441–447. [Google Scholar] [CrossRef] [Green Version]
- El-Nezami, H.; Kankaanpaa, P.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 1998, 36, 321–326. [Google Scholar] [CrossRef]
- Pierides, M.; El-Nezami, H.; Peltonen, K.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Protect. 2000, 63, 645–650. [Google Scholar] [CrossRef]
- Turbic, A.; Ahokas, J.T.; Haskard, C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002, 19, 144–152. [Google Scholar] [CrossRef]
- Kabak, B.; Var, I. Binding of aflatoxin M1 by Lactobacillus and Bifidobacterium strains. Milchwissenschaft 2004, 59, 301–303. [Google Scholar]
- Khattab, A.A.; Ibrahim, M.I.M.; El-Kady, A.A. Ochratoxin A biosorption onto genetically improved of Lactobacillus delbrueckii mutants. Int. Food. Res. J. 2018, 25, 515–522. [Google Scholar]
- Liu, J.; Pan, D.; Wu, X.; Chen, H.; Cao, H.; Li, Q.X.; Hua, R. Enhanced degradation of prometryn and other s-triazine herbicides in pure cultures and wastewater by polyvinyl alcohol-sodium alginate immobilized Leucobacter sp. JW-1. Sci. Total Environ. 2018, 615, 78–86. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, M.S.; Siddavattam, D.; Karegoudar, T.B. Generation of continuous packed bed reactor with PVA-alginate blend immobilized Ochrobactrum sp. DGVK1 cells for effective removal of N,N-dimethylformamide from industrial effluents. J. Hazard. Mater. 2012, 199, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Farbo, M.G.; Urgeghea, P.P.; Fioria, S.; Marceddu, S.; Jaoua, S.; Migheli, Q. Adsorption of ochratoxin A from grape juice by yeast cells immobilised in calcium alginate beads. Int. J. Food Microbiol. 2016, 217, 29–34. [Google Scholar] [CrossRef]
- Shibayama, M.; Sato, M.; Kimura, Y.; Fujiwara, H.; Nomura, S. 11B n.m.r. study on the reaction of poly(vinyl alcohol) with boric acid. Polymer 1988, 29, 336–340. [Google Scholar] [CrossRef]
- Hansen, E.W.; Holm, K.H.; Jahr, D.M.; Olafsen, K.; Stori, A. Reaction of poly(vinyl alcohol) and dialdehydes during gel formation probed by 1H n.m.r.—A kinetic study. Polymer 1997, 38, 4863–4871. [Google Scholar] [CrossRef]
- Yoon, J.; Oh, D.X.; Jo, C.; Lee, J.; Hwang, D.S. Improvement of desolvation and resilience of alginate binders for Si-based anodes in a lithium ion battery by calcium-mediated cross-linking. Phys. Chem. Chem. Phys. 2014, 16, 25628–25635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Huang, Y.; Cai, Z.; Cong, W.; Ouyang, F. Immobilization of Acidithiobacillus ferrooxidans by a PVA–boric acid method for ferrous sulphate oxidation. Process Biochem. 2004, 39, 2129–2133. [Google Scholar] [CrossRef]
- Castro, R.I.; Morales-Quintana, L.; Alvarado, N.; Guzmán, L.; Forero-Doria, O.; Valenzuela-Riffo, F.; Laurie, V.F. Design and optimization of a self-assembling complex based on microencapsulated calcium alginate and glutathione (CAG) using response surface methodology. Polymers 2021, 13, 2080. [Google Scholar] [CrossRef]
- Rodríguez, Y.A.N.; Castro, R.I.; Arenas, F.A.; López-Cabaña, Z.E.; Carreño, G.; Carrasco-Sánchez, V.; Marican, A.; Villaseñor, J.; Vargas, E.; Santos, L.S.; et al. Preparation of Hydrogel/silver nanohybrids mediated by tunable-size silver nanoparticles for potential antibacterial applications. Polymers 2019, 11, 716. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, Z.; Shi, D.; Zhou, T.; Kaneko, D.; Chen, M. High strength and toughness of double physically cross-linked hydrogels composed of polyvinyl alcohol and calcium alginate. J. Appl. Polym. Sci. 2021, 138, 49987. [Google Scholar] [CrossRef]
- Del Prete, V.; Rodriguez, H.; Carrascosa, A.V.; de las Rivas, B.; Garcia-Moruno, E.; Munoz, R. In vitro removal of ochratoxin a by wine lactic acid bacteria. J. Food. Prot. 2007, 70, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Golinski, P.; Bryla, M.; Waskiewicz, A. The efficiency of lactic acid bacteria against pathogenic fungi and mycotoxins. Arh. Hig. Rada. Toksikol. 2018, 69, 32–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, M. The adsorption of ohratoxin A by Lactobacillus Species. Toxins 2014, 6, 2826–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasov, N.; Jaric, S.; Kireev, D.; Emelianov, A.V.; Orlov, A.V.; Gadjanski, I.; Nikitin, P.I.; Akinwande, D.; Bobrinetskiy, I. Real-time detection of ochratoxin A in wine through insight of aptamer conformation in conjunction with graphene field-effect transistor. Biosen. Bioelectron. 2022, 200, 113890. [Google Scholar] [CrossRef]
- Sangsila, A.; Faucet-Marquis, V.; Pfohl-Leszkowicz, A.; Itsaranuwat, P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Control. 2016, 62, 187–192. [Google Scholar] [CrossRef]
- Many, D.A.; Ibrahim, A.S.S.; El-Diwany, A.I. Biosynthesis of alkaline protease by alkaliphilic Bacillus sp. NPST-AK15 cells immobilized in gel matrices. Egypt. Pharm. J. 2019, 18, 201–207. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Determination of total phenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1.1.1–I1.1.8. [Google Scholar]
- Carrasco-Sánchez, V.; Marican, A.; Vergara-Jaque, A.; Folch-Cano, C.; Comer, J.; Laurie, V.F. Polymeric substances for the removal of ochratoxin A from red wine followed by computational modeling of the complexes formed. Food Chem. 2018, 265, 159–164. [Google Scholar] [CrossRef]





| Complexes | PVA | LP Concentration in Wet Weight |
|---|---|---|
| AP−1 | 5% Mowiol 6–98 | - |
| APLP−1 | 5% Mowiol 6–98 | 1% |
| APLP−1b | 5% Mowiol 6–98 | 2% |
| AP-2 | 5% Mowiol 10–98 | - |
| APLP-2 | 5% Mowiol 10–98 | 1% |
| APLP-2b | 5% Mowiol 10–98 | 2% |
| Uncodified | Codified | Results | ||||||
|---|---|---|---|---|---|---|---|---|
| Complexes | PVA (Mw) | LAB (%) | Time (min) | Mw PVA | % LAB | Contact Time | OTA Removal (%) | Unremoved Phenols (%) |
| AP−1 | 47,000 | 0 | 15 | −1 | −1 | −1 | 32.22 ± 0.71 | 72.23 ± 0.61 |
| APLP−1 | 47,000 | 1 | 15 | −1 | 0 | −1 | 38.99 ± 0.12 | 67.50 ± 1.65 |
| APLP−1b | 47,000 | 2 | 15 | −1 | 1 | −1 | 40.40 ± 0.79 | 68.13 ± 1.20 |
| AP-2 | 61,000 | 0 | 15 | 1 | −1 | −1 | 33.94 ± 0.43 | 64.53 ± 0.49 |
| APLP-2 | 61,000 | 1 | 15 | 1 | 0 | −1 | 40.52 ± 0.53 | 62.40 ± 1.41 |
| APLP-2b | 61,000 | 2 | 15 | 1 | 1 | −1 | 44.92 ± 0.31 | 62.41 ± 0.78 |
| AP−1 | 47,000 | 0 | 30 | −1 | −1 | −0.66 | 39.46 ± 0.28 | 67.73 ± 0.97 |
| APLP−1 | 47,000 | 1 | 30 | −1 | 0 | −0.66 | 45.45 ± 0.44 | 67.23 ± 1.26 |
| APLP−1b | 47,000 | 2 | 30 | −1 | 1 | −0.66 | 45.25 ± 0.26 | 66.43 ± 2.29 |
| AP-2 | 61,000 | 0 | 30 | 1 | −1 | −0.66 | 34.69 ± 0.29 | 62.90 ± 0.70 |
| APLP-2 | 61,000 | 1 | 30 | 1 | 0 | −0.66 | 42.62 ± 0.40 | 59.17 ± 0.60 |
| APLP-2b | 61,000 | 2 | 30 | 1 | 1 | −0.66 | 47.59 ± 0.36 | 59.03 ± 0.57 |
| AP−1 | 47,000 | 0 | 60 | −1 | −1 | 1 | 45.64 ± 0.11 | 64.17 ± 0.45 |
| APLP−1 | 47,000 | 1 | 60 | −1 | 0 | 1 | 48.48 ± 0.38 | 65.03 ± 0.64 |
| APLP−1b | 47,000 | 2 | 60 | −1 | 1 | 1 | 50.87 ± 0.65 | 64.77 ± 0.51 |
| AP-2 | 61,000 | 0 | 60 | 1 | −1 | 1 | 41.71 ± 0.82 | 61.83 ± 1.12 |
| APLP-2 | 61,000 | 1 | 60 | 1 | 0 | 1 | 50.91 ± 0.68 | 58.00 ± 0.46 |
| APLP-2b | 61,000 | 2 | 60 | 1 | 1 | 1 | 55.53 ± 0.79 | 59.30 ± 0.82 |
| Source | Sum of Squares | Degree’s Liberty | Mean Square | Razon-F | Valor-P | |
|---|---|---|---|---|---|---|
| OTA removal | A: Factor A | 762.057 | 1 | 762.057 | 418.34 | 0.0000 |
| B: Factor B | 965.55 | 1 | 965.55 | 530.05 | 0.0000 | |
| C: Factor C | 5.12154 | 1 | 5.12154 | 2.81 | 0.1008 | |
| AA | 39.215 | 1 | 39.215 | 21.53 | 0.0000 | |
| AB | 0.00207382 | 1 | 0.00207382 | 0.00 | 0.9732 | |
| AC | 85.3931 | 1 | 85.3931 | 46.88 | 0.0000 | |
| BB | 53.0585 | 1 | 53.0585 | 29.13 | 0.0000 | |
| BC | 0.00192448 | 1 | 0.00192448 | 0.00 | 0.9742 | |
| Blocks | 0.0981739 | 2 | 0.0490869 | 0.03 | 0.9734 | |
| Total error | 78.3301 | 43 | 1.82163 | |||
| Unremoved phenols | A: Factor A | 33.611 | 1 | 33.611 | 21.86 | 0.0000 |
| B: Factor B | 145.203 | 1 | 145.203 | 94.45 | 0.0000 | |
| C: Factor C | 426.116 | 1 | 426.116 | 277.17 | 0.0000 | |
| AA | 18.2533 | 1 | 18.2533 | 11.87 | 0.0013 | |
| AB | 7.47136 | 1 | 7.47136 | 4.86 | 0.0329 | |
| AC | 3.48444 | 1 | 3.48444 | 2.27 | 0.1395 | |
| BB | 32.7231 | 1 | 32,7231 | 21.28 | 0.0000 | |
| BC | 6.11208 | 1 | 6.11208 | 3.98 | 0.0525 | |
| Blocks | 4.77778 | 2 | 2.38889 | 1.55 | 0.2231 | |
| Total error | 66.108 | 43 | 1.5374 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, R.I.; Laurie, V.F.; Padilla, C.; Carrasco-Sánchez, V. Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study. Toxins 2022, 14, 230. https://doi.org/10.3390/toxins14040230
Castro RI, Laurie VF, Padilla C, Carrasco-Sánchez V. Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study. Toxins. 2022; 14(4):230. https://doi.org/10.3390/toxins14040230
Chicago/Turabian StyleCastro, Ricardo Ignacio, V. Felipe Laurie, Carlos Padilla, and Verónica Carrasco-Sánchez. 2022. "Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study" Toxins 14, no. 4: 230. https://doi.org/10.3390/toxins14040230
APA StyleCastro, R. I., Laurie, V. F., Padilla, C., & Carrasco-Sánchez, V. (2022). Removal of Ochratoxin A from Red Wine Using Alginate-PVA-L. plantarum (APLP) Complexes: A Preliminary Study. Toxins, 14(4), 230. https://doi.org/10.3390/toxins14040230