Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure—A Tri-National Cohort
Abstract
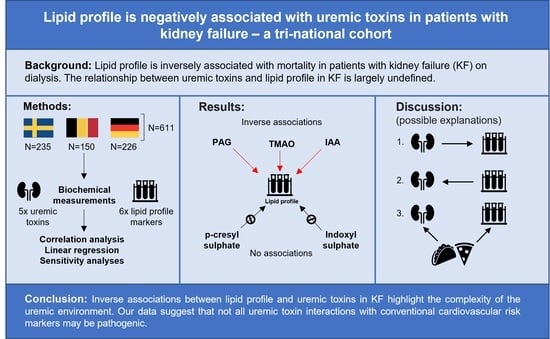
:1. Introduction
2. Results
2.1. Baseline Characteristics
2.2. Univariate Correlations of Lipid Profile and Uremic Toxins
2.3. Multivariate Regression Analyses
2.4. Sensitivity Analyses
3. Discussion
4. Materials and Methods
4.1. Patients and Study Design
4.2. Biochemical Analysis/Clinical Parameters
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kooman, J.P.; Kotanko, P.; Schols, A.M.W.J.; Shiels, P.G.; Stenvinkel, P. Chronic Kidney Disease and Premature Ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Ebert, T.; Neytchev, O.; Witasp, A.; Kublickiene, K.; Stenvinkel, P.; Shiels, P.G. Inflammation and Oxidative Stress in Chronic Kidney Disease and Dialysis Patients. Antioxid. Redox Signal. 2021, 35, 1426–1448. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721–729. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; Hordijk, P.L.; Vervloet, M.G. Most Exposed: The Endothelium in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2020, 35, 1478–1487. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on Uremic Toxins: Classification, Concentration, and Interindividual Variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Rosner, M.H.; Reis, T.; Husain-Syed, F.; Vanholder, R.; Hutchison, C.; Stenvinkel, P.; Blankestijn, P.J.; Cozzolino, M.; Juillard, L.; Kashani, K.; et al. Classification of Uremic Toxins and Their Role in Kidney Failure. Clin. J. Am. Soc. Nephrol. 2021, 16, 1918–1928. [Google Scholar] [CrossRef]
- Leong, S.C.; Sirich, T.L. Indoxyl Sulfate—Review of Toxicity and Therapeutic Strategies. Toxins 2016, 8, 358. [Google Scholar] [CrossRef]
- Opdebeeck, B.; Maudsley, S.; Azmi, A.; Maré, A.D.; Leger, W.D.; Meijers, B.; Verhulst, A.; Evenepoel, P.; D’Haese, P.C.; Neven, E. Indoxyl Sulfate and P-Cresyl Sulfate Promote Vascular Calcification and Associate with Glucose Intolerance. J. Am. Soc. Nephrol. 2019, 30, 751–766. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A.; (EUTox) on behalf of the E.U.T.W.G. Serum Indoxyl Sulfate Is Associated with Vascular Disease and Mortality in Chronic Kidney Disease Patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lano, G.; Burtey, S.; Sallée, M. Indoxyl Sulfate, a Uremic Endotheliotoxin. Toxins 2020, 12, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Tsuruoka, S.; Ioka, T.; Ando, H.; Ito, C.; Akimoto, T.; Fujimura, A.; Asano, Y.; Kusano, E. Indoxyl Sulfate Stimulates Proliferation of Rat Vascular Smooth Muscle Cells. Kidney Int. 2006, 69, 1780–1785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Q.; Zheng, B.; Liu, N.; Liu, J.; Liu, W.; Huang, X.; Zeng, X.; Chen, L.; Li, Z.; Ouyang, D. Trimethylamine N-Oxide Exacerbates Renal Inflammation and Fibrosis in Rats With Diabetic Kidney Disease. Front. Physiol. 2021, 12, 896. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [Green Version]
- Noels, H.; Lehrke, M.; Vanholder, R.; Jankowski, J. Lipoproteins and Fatty Acids in Chronic Kidney Disease: Molecular and Metabolic Alterations. Nat. Rev. Nephrol. 2021, 17, 528–542. [Google Scholar] [CrossRef]
- Barbagallo, C.M.; Cefalù, A.B.; Giammanco, A.; Noto, D.; Caldarella, R.; Ciaccio, M.; Averna, M.R.; Nardi, E. Lipoprotein Abnormalities in Chronic Kidney Disease and Renal Transplantation. Life 2021, 11, 315. [Google Scholar] [CrossRef]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric Dimethylarginine, High-Density Lipoproteins and Cardiovascular Disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef]
- Ebert, T.; Qureshi, A.R.; Lamina, C.; Fotheringham, J.; Froissart, M.; Eckardt, K.-U.; Wheeler, D.C.; Floege, J.; Kronenberg, F.; Stenvinkel, P. Time-Dependent Lipid Profile Inversely Associates with Mortality in Hemodialysis Patients—Independent of Inflammation/Malnutrition. J. Intern. Med. 2021, 290, 910–921. [Google Scholar] [CrossRef]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The Cardiovascular Effect of the Uremic Solute Indole-3 Acetic Acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poesen, R.; Claes, K.; Evenepoel, P.; de Loor, H.; Augustijns, P.; Kuypers, D.; Meijers, B. Microbiota-Derived Phenylacetylglutamine Associates with Overall Mortality and Cardiovascular Disease in Patients with CKD. J. Am. Soc. Nephrol. 2016, 27, 3479–3487. [Google Scholar] [CrossRef] [Green Version]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Kuypers, D.; Augustijns, P.; Meijers, B. Metabolism, Protein Binding, and Renal Clearance of Microbiota–Derived p-Cresol in Patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid Management in Patients with Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Fu, Q.; Hui Wang, B.; Jin, W.; Li, Z. Indoxyl Sulfate Stimulates Oxidized LDL Uptake through Up-Regulation of CD36 Expression in THP-1 Macrophages. J. Appl. Biomed. 2014, 12, 203–209. [Google Scholar] [CrossRef]
- Matsuo, K.; Yamamoto, S.; Wakamatsu, T.; Takahashi, Y.; Kawamura, K.; Kaneko, Y.; Goto, S.; Kazama, J.J.; Narita, I. Increased Proinflammatory Cytokine Production and Decreased Cholesterol Efflux Due to Downregulation of ABCG1 in Macrophages Exposed to Indoxyl Sulfate. Toxins 2015, 7, 3155–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 2062. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.; Pawelzik, S.-C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [Green Version]
- Massy, Z.A. The Role of Lipids and Uremic Toxins in Cardiovascular Disease in CKD. Clin. Exp. Nephrol. 2014, 18, 255–256. [Google Scholar] [CrossRef]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C.O. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins 2016, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Qiu, J.; Lian, J.; Yang, X.; Zhou, J. Gut Metabolite Trimethylamine-N-Oxide in Atherosclerosis: From Mechanism to Therapy. Front. Cardiovasc. Med. 2021, 8, 1560. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Ling, A.V.; Manthena, P.V.; Gearing, M.E.; Graham, M.J.; Crooke, R.M.; Croce, K.J.; Esquejo, R.M.; Clish, C.B.; Vicent, D.; et al. Flavin-Containing Monooxygenase 3 as a Potential Player in Diabetes-Associated Atherosclerosis. Nat. Commun. 2015, 6, 6498. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.-H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149. [Google Scholar] [CrossRef]
- Villette, R.; Kc, P.; Beliard, S.; Salas Tapia, M.F.; Rainteau, D.; Guerin, M.; Lesnik, P. Unraveling Host-Gut Microbiota Dialogue and Its Impact on Cholesterol Levels. Front. Pharmacol. 2020, 11, 278. [Google Scholar] [CrossRef]
- Rauchhaus, M.; Coats, A.J.; Anker, S.D. The Endotoxin-Lipoprotein Hypothesis. Lancet 2000, 356, 930–933. [Google Scholar] [CrossRef]
- Liu, Y.; Coresh, J.; Eustace, J.A.; Longenecker, J.C.; Jaar, B.; Fink, N.E.; Tracy, R.P.; Powe, N.R.; Klag, M.J. Association Between Cholesterol Level and Mortality in Dialysis Patients: Role of Inflammation and Malnutrition. JAMA 2004, 291, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Skipper, M. Genetic Determinants of Lipid Profiles. Nat. Rev. Genet. 2008, 9, 164. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Luttropp, K.; McGuinness, D.; Witasp, A.; Qureshi, A.R.; Wernerson, A.; Nordfors, L.; Schalling, M.; Ripsweden, J.; Wennberg, L.; et al. CDKN2A/P16INK4a Expression Is Associated with Vascular Progeria in Chronic Kidney Disease. Aging 2017, 9, 494–507. [Google Scholar] [CrossRef] [Green Version]
- Tönjes, A.; Hoffmann, A.; Kralisch, S.; Qureshi, A.R.; Klöting, N.; Scholz, M.; Schleinitz, D.; Bachmann, A.; Kratzsch, J.; Nowicki, M.; et al. Pro-Neurotensin Depends on Renal Function and Is Related to All-Cause Mortality in Chronic Kidney Disease. Eur. J. Endocrinol. 2020, 183, 233–244. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Blank, D.; Zakarian, R.; Bergeron, J.; Frohlich, J. Triglycerides and Small Dense LDL: The Twin Achilles Heels of the Friedewald Formula. Clin. Biochem. 2003, 36, 499–504. [Google Scholar] [CrossRef]
- Tremblay, A.J.; Morrissette, H.; Gagné, J.-M.; Bergeron, J.; Gagné, C.; Couture, P. Validation of the Friedewald Formula for the Determination of Low-Density Lipoprotein Cholesterol Compared with β-Quantification in a Large Population. Clin. Biochem. 2004, 37, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Anette, V.; Marianne, B.; Anne, T.; Nordestgaard Børge, G. Elevated Remnant Cholesterol Causes Both Low-Grade Inflammation and Ischemic Heart Disease, Whereas Elevated Low-Density Lipoprotein Cholesterol Causes Ischemic Heart Disease Without Inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y. (Lucy); Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- De Loor, H.; Poesen, R.; De Leger, W.; Dehaen, W.; Augustijns, P.; Evenepoel, P.; Meijers, B. A Liquid Chromatography—Tandem Mass Spectrometry Method to Measure a Selected Panel of Uremic Retention Solutes Derived from Endogenous and Colonic Microbial Metabolism. Anal. Chim. Acta 2016, 936, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Fitzpatrick, J.; Winkler, C.A.; Binns-Roemer, E.A.; Corona-Villalobos, C.P.; Jaar, B.G.; Sozio, S.M.; Parekh, R.S.; Estrella, M.M. APOL1 Risk Variants and Subclinical Cardiovascular Disease in Incident Hemodialysis Patients. Kidney Int. Rep. 2021, 6, 333–341. [Google Scholar] [CrossRef] [PubMed]

| Entire Cohort | Female Subjects | Male Subjects | p | Dialysis | Non-Dialysis | p | |
|---|---|---|---|---|---|---|---|
| N | 611 | 212 | 399 | - | 423 | 188 | - |
| Age (years) | 55 (43–67) | 56 (44–68) | 54 (42–65) | 0.057 | 55 (43–65) | 56 (42–70) | 0.061 |
| Male sex (N [%]) | 399 [65.3%] | - | - | - | 287 [67.8%] | 112 [59.6%] | 0.047 |
| BMI (kg/m2) | 24.8 (22.6–27.5) | 24.8 (22–28) | 24.8 (22.9–27.4) | 0.572 | 24.6 (22.5–27.8) | 25.1 (23.1–27.3) | 0.468 |
| SBP (mmHg) | 140 (126–153) | 140 (125–154) | 139 (126–153) | 0.751 | 139 (121–153) | 140 (129–152) | 0.132 |
| DBP (mmHg) | 80 (72–90) | 80 (71–90) | 80 (72–90) | 0.408 | 80 (70–90) | 82 (75–90) | 0.029 |
| Diabetes mellitus (N [%]) | 53 [8.7%] | 16 [7.5%] | 37 [9.3%] | 0.422 | 50 [12.0%] | 3 [1.6%] | <0.001 |
| Statin users (N [%]) | 231 [37.8%] | 79 [37.3%] | 152 [38.1%] | 0.905 | 156 [39.8%] | 75 [40.1%] | 0.787 |
| Dialysis (N [%]) | 423 [69.2%] | 136 [64.2%] | 287 [71.9%] | 0.050 | 423 [100%] | 0 [0%] | - |
| Vintage (months) | 16 (3–43) | 18 (5–45) | 16 (3–43) | 0.688 | 24 (11–47) | - | - |
| Creatinine (µmol/L) | 637 (427–848) | 541 (349–725) | 700 (500–911) | <0.001 | 706 (556–901) | 356 (172–631) | <0.001 |
| eGFR (mL/min/1.73 m2) | 7 (5–11) | 7 (5–12) | 7 (5–11) | 0.658 | 6 (5–8) | 13 (7–28) | <0.001 |
| Albumin (g/L) | 38 (33–42) | 37 (33–42) | 38 (34–42) | 0.076 | 38 (34–42) | 37 (22–40) | 0.040 |
| hsCRP (mg/L) | 2.0 (0.9–4.9) | 1.9 (0.9–5.9) | 2.0 (0.8–4.4) | 0.416 | 2.2 (1.0–5.9) | 1.4 (0.7–3.4) | <0.001 |
| Total chol. (mmol/L) | 4.6 (3.9–5.5) | 4.9 (4.2–6.2) | 4.4 (3.7–5.2) | <0.001 | 4.5 (3.8–5.3) | 4.9 (4.1–6.3) | <0.001 |
| HDL chol. (mmol/L) | 1.3 (1.0–1.7) | 1.5 (1.2–1.8) | 1.2 (1.0–1.5) | <0.001 | 1.3 (1.0–1.6) | 1.4 (1.1–1.7) | 0.009 |
| Non-HDL chol (mmol/L) | 3.2 (2.5–4.1) | 3.4 (2.7–4.6) | 3.2 (2.4–3.9) | 0.002 | 3.1 (2.4–3.9) | 3.6 (2.7–4.7) | <0.001 |
| LDL chol. (mmol/L) | 2.5 (1.8–3.3) | 2.6 (2.0–3.7) | 2.4 (1.8–3.1) | <0.001 | 2.3 (1.8–3.0) | 2.8 (2.1–3.8) | <0.001 |
| Remnant chol. (mmol/L) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.592 | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 0.675 |
| Triglycerides (mmol/L) | 1.4 (1.0–2.0) | 1.4 (1.0–2.0) | 1.5 (1.1–2.0) | 0.630 | 1.4 (1.0–2.1) | 1.5 (1.0–2.0) | 0.749 |
| Indoxyl sulphate (µmol/L) | 87.8 (43.1–135.4) | 80.5 (31.4–126.6) | 92.8 (54.1–143.9) | 0.005 | 106.4 (73.0–154.3) | 27.9 (12.7–74.9) | <0.001 |
| p-cresyl sulphate (µmol/L) | 133.4 (68.0–202.7) | 127.7 (69.2–203.0) | 137 (67.2–202.3) | 0.615 | 146.2 (85.8–207.6) | 101.1 (45.4–187.1) | <0.001 |
| Indole-3 acetic acid (µmol/L) | 4.5 (3.0–6.3) | 4.3 (2.7–5.9) | 4.6 (3.1–6.6) | 0.139 | 5.0 (3.4–7.1) | 3.3 (2.4–4.6) | <0.001 |
| TMAO (µmol/L) | 57.6 (27.9–105.5) | 54.6 (24.1–118.1) | 60.2 (29.8–104.5) | 0.514 | 71.4 (41.0–123.0) | 25.9 (10.7–65.3) | <0.001 |
| Phenylacetylglutamine (µmol/L) | 46.1 (18.9–95.9) | 45.6 (15.3–110.3) | 46.5 (20.9–89.4) | 0.936 | 71.5 (32.0–126.0) | 13.0 (5.6–33.2) | <0.001 |
| Analytes (µmol/L) | Total Cholesterol (mmol/L) | HDL Cholesterol (mmol/L) | Non-HDL Cholesterol (mmol/L) | LDL Cholesterol (mmol/L) | Remnant Cholesterol (mmol/L) | Triglycerides (mmol/L) | |
|---|---|---|---|---|---|---|---|
| Indoxyl sulphate | ꞵ | −0.041 | −0.009 | −0.024 | −0.018 | - | - |
| p | 0.164 | 0.742 | 0.404 | 0.551 | - | - | |
| p-cresyl sulphate | ꞵ | −0.016 | − | −0.007 | 0.038 | - | - |
| p | 0.747 | − | 0.885 | 0.448 | - | - | |
| Indole-3 acetic acid | ꞵ | −0.091 | − | −0.150 | −0.122 | - | - |
| p | 0.090 | − | 0.004 | 0.025 | - | - | |
| TMAO | ꞵ | −0.051 | − | −0.079 | −0.045 | - | - |
| p | 0.165 | − | 0.031 | 0.229 | - | - | |
| Phenylacetylglutamine | ꞵ | −0.095 | − | −0.133 | −0.071 | - | - |
| p | <0.001 | − | <0.001 | 0.016 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobson, S.; de Loor, H.; Kublickiene, K.; Beige, J.; Evenepoel, P.; Stenvinkel, P.; Ebert, T. Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure—A Tri-National Cohort. Toxins 2022, 14, 412. https://doi.org/10.3390/toxins14060412
Hobson S, de Loor H, Kublickiene K, Beige J, Evenepoel P, Stenvinkel P, Ebert T. Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure—A Tri-National Cohort. Toxins. 2022; 14(6):412. https://doi.org/10.3390/toxins14060412
Chicago/Turabian StyleHobson, Sam, Henriette de Loor, Karolina Kublickiene, Joachim Beige, Pieter Evenepoel, Peter Stenvinkel, and Thomas Ebert. 2022. "Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure—A Tri-National Cohort" Toxins 14, no. 6: 412. https://doi.org/10.3390/toxins14060412
APA StyleHobson, S., de Loor, H., Kublickiene, K., Beige, J., Evenepoel, P., Stenvinkel, P., & Ebert, T. (2022). Lipid Profile Is Negatively Associated with Uremic Toxins in Patients with Kidney Failure—A Tri-National Cohort. Toxins, 14(6), 412. https://doi.org/10.3390/toxins14060412