Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner
Abstract
:1. Introduction
2. Results
2.1. Effects of L2 and BNP on RAW264.7 Cell Viability
2.2. Effects of L2 and BNP on LPS-Induced Inflammatory Cytokines
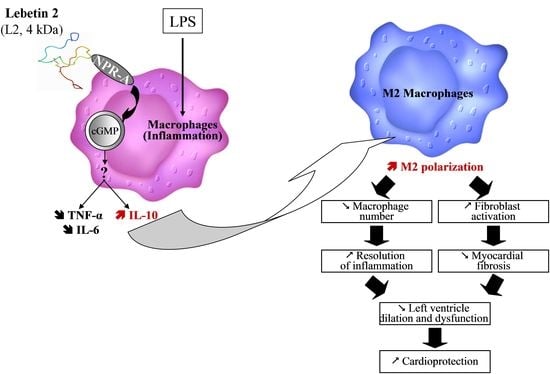
2.3. Effects of L2 and BNP on Macrophage Polarization and Related Mechanisms
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Drugs and Reagents
5.2. Experimental Research Design
5.3. Cell Culture
5.4. MTT Assay Protocol for Cell Viability
5.5. ELISA Assay for Inflammatory Cytokine Evaluation
5.6. Flow Cytometry for Macrophage Polarization Assessment
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tourki, B.; Dumesnil, A.; Belaidi, E.; Ghrir, S.; Godin-Ribuot, D.; Marrakchi, N.; Richard, V.; Mulder, P.; Messadi, E. Lebetin 2, a Snake Venom-Derived B-Type Natriuretic Peptide, Provides Immediate and Prolonged Protection against Myocardial Ischemia-Reperfusion Injury via Modulation of Post-Ischemic Inflammatory Response. Toxins 2019, 11, 524. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.B.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Gulati, R.; Mueske, C.S.; Witt, T.A.; Lerman, A.; Burnett, J.C., Jr.; Simari, R.D. Gene transfer of a novel vasoactive natriuretic peptide stimulates cGMP and lowers blood pressure in mice. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2213–H2218. [Google Scholar] [CrossRef] [PubMed]
- Lyu, T.; Zhao, Y.; Zhang, T.; Zhou, W.; Yang, F.; Ge, H.; Ding, S.; Pu, J.; He, B. Natriuretic peptides as an adjunctive treatment for acute myocardial infarction: Insights from the meta-analysis of 1,389 patients from 20 trials. Int. Heart J. 2014, 55, 8–16. [Google Scholar] [CrossRef]
- Wu, B.; Jiang, H.; Lin, R.; Cui, B.; Wen, H.; Lu, Z. Pretreatment with B-type natriuretic peptide protects the heart from ischemia-reperfusion injury by inhibiting myocardial apoptosis. Tohoku J. Exp. Med. 2009, 219, 107–114. [Google Scholar] [CrossRef]
- Ren, B.; Shen, Y.; Shao, H.; Qian, J.; Wu, H.; Jing, H. Brain natriuretic peptide limits myocardial infarct size dependent of nitric oxide synthase in rats. Clin. Chim. Acta 2007, 377, 83–87. [Google Scholar] [CrossRef]
- Ren, B.; Wu, H.; Yin, R.; Xu, L.; Jing, H.; Li, M.; Jiang, F.; Wang, Z. B-type natriuretic peptide pretreatment attenuates heart ischemia-reperfusion injury in rats. Transplant. Proc. 2010, 42, 4496–4498. [Google Scholar] [CrossRef]
- Pagel-Langenickel, I.; Buttgereit, J.; Bader, M.; Langenickel, T.H. Natriuretic peptide receptor B signaling in the cardiovascular system: Protection from cardiac hypertrophy. J. Mol. Med. 2007, 85, 797–810. [Google Scholar] [CrossRef]
- Breivik, L.; Jensen, A.; Guvag, S.; Aarnes, E.K.; Aspevik, A.; Helgeland, E.; Hovland, S.; Brattelid, T.; Jonassen, A.K. B-type natriuretic peptide expression and cardioprotection is regulated by Akt dependent signaling at early reperfusion. Peptides 2015, 66, 43–50. [Google Scholar] [CrossRef]
- D’Souza, S.P.; Yellon, D.M.; Martin, C.; Schulz, R.; Heusch, G.; Onody, A.; Ferdinandy, P.; Baxter, G.F. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1592–H1600. [Google Scholar] [CrossRef] [PubMed]
- Tourki, B.; Mateo, P.; Morand, J.; Elayeb, M.; Godin-Ribuot, D.; Marrakchi, N.; Belaidi, E.; Messadi, E. Lebetin 2, a Snake Venom-Derived Natriuretic Peptide, Attenuates Acute Myocardial Ischemic Injury through the Modulation of Mitochondrial Permeability Transition Pore at the Time of Reperfusion. PLoS ONE 2016, 11, e0162632. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Vollmar, A.M. Effects of different natriuretic peptides on nitric oxide synthesis in macrophages. Endocrinology 1997, 138, 4282–4290. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.M.; Schulz, R. Expression and differential regulation of natriuretic peptides in mouse macrophages. J. Clin. Investig. 1995, 95, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Huang, X.; Zhang, K.; Jiang, H.; Hu, X. Anti-inflammatory effect of B-type natriuretic peptide postconditioning during myocardial ischemia-reperfusion: Involvement of PI3K/Akt signaling pathway. Inflammation 2014, 37, 1669–1674. [Google Scholar] [CrossRef]
- Kapoun, A.M.; Liang, F.; O’Young, G.; Damm, D.L.; Quon, D.; White, R.T.; Munson, K.; Lam, A.; Schreiner, G.F.; Protter, A.A. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: Fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ. Res. 2004, 94, 453–461. [Google Scholar] [CrossRef]
- Izumi, T.; Saito, Y.; Kishimoto, I.; Harada, M.; Kuwahara, K.; Hamanaka, I.; Takahashi, N.; Kawakami, R.; Li, Y.; Takemura, G.; et al. Blockade of the natriuretic peptide receptor guanylyl cyclase-A inhibits NF-kappaB activation and alleviates myocardial ischemia/reperfusion injury. J. Clin. Investig. 2001, 108, 203–213. [Google Scholar] [CrossRef]
- Kawakami, R.; Saito, Y.; Kishimoto, I.; Harada, M.; Kuwahara, K.; Takahashi, N.; Nakagawa, Y.; Nakanishi, M.; Tanimoto, K.; Usami, S.; et al. Overexpression of brain natriuretic peptide facilitates neutrophil infiltration and cardiac matrix metalloproteinase-9 expression after acute myocardial infarction. Circulation 2004, 110, 3306–3312. [Google Scholar] [CrossRef]
- Barbouche, R.; Marrakchi, N.; Mansuelle, P.; Krifi, M.; Fenouillet, E.; Rochat, H.; el Ayeb, M. Novel anti-platelet aggregation polypeptides from Vipera lebetina venom: Isolation and characterization. FEBS Lett. 1996, 392, 6–10. [Google Scholar] [CrossRef]
- Vink, S.; Jin, A.H.; Poth, K.J.; Head, G.A.; Alewood, P.F. Natriuretic peptide drug leads from snake venom. Toxicon 2012, 59, 434–445. [Google Scholar] [CrossRef]
- Allaoui, H.; Rached, N.; Marrakchi, N.; Cherif, A.; Mosbah, A.; Messadi, E. In Silico Study of the Mechanisms Underlying the Action of the Snake Natriuretic-Like Peptide Lebetin 2 during Cardiac Ischemia. Toxins 2022, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- Weirather, J.; Hofmann, U.D.; Beyersdorf, N.; Ramos, G.C.; Vogel, B.; Frey, A.; Ertl, G.; Kerkau, T.; Frantz, S. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014, 115, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, M.; Zhong, C.; Xu, B.; Kang, L. M2-like macrophages transplantation protects against the doxorubicin-induced heart failure via mitochondrial transfer. Biomater. Res. 2022, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Pittet, M.J.; Swirski, F.K. Monocytes: Protagonists of infarct inflammation and repair after myocardial infarction. Circulation 2010, 121, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, K.; Kaikita, K.; Hayasaki, T.; Honda, T.; Kobayashi, H.; Sakashita, N.; Suzuki, H.; Kodama, T.; Ogawa, H.; Takeya, M. Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation 2007, 115, 1904–1911. [Google Scholar] [CrossRef]
- Shiraishi, M.; Shintani, Y.; Shintani, Y.; Ishida, H.; Saba, R.; Yamaguchi, A.; Adachi, H.; Yashiro, K.; Suzuki, K. Alternatively activated macrophages determine repair of the infarcted adult murine heart. J. Clin. Investig. 2016, 126, 2151–2166. [Google Scholar] [CrossRef]
- Dayan, V.; Yannarelli, G.; Billia, F.; Filomeno, P.; Wang, X.H.; Davies, J.E.; Keating, A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res. Cardiol. 2011, 106, 1299–1310. [Google Scholar] [CrossRef]
- Degboe, Y.; Rauwel, B.; Baron, M.; Boyer, J.F.; Ruyssen-Witrand, A.; Constantin, A.; Davignon, J.L. Polarization of Rheumatoid Macrophages by TNF Targeting Through an IL-10/STAT3 Mechanism. Front. Immunol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Jing, R.; Long, T.Y.; Pan, W.; Li, F.; Xie, Q.Y. IL-6 knockout ameliorates myocardial remodeling after myocardial infarction by regulating activation of M2 macrophages and fibroblast cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6283–6291. [Google Scholar]
- Barsig, J.; Kusters, S.; Vogt, K.; Volk, H.D.; Tiegs, G.; Wendel, A. Lipopolysaccharide-induced interleukin-10 in mice: Role of endogenous tumor necrosis factor-alpha. Eur. J. Immunol. 1995, 25, 2888–2893. [Google Scholar] [CrossRef]
- Lopes, R.L.; Borges, T.J.; Zanin, R.F.; Bonorino, C. IL-10 is required for polarization of macrophages to M2-like phenotype by mycobacterial DnaK (heat shock protein 70). Cytokine 2016, 85, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.; Hung, M.E.; Cangelose, B.K.; Leonard, J.N. Regulation of the IL-10-driven macrophage phenotype under incoherent stimuli. Innate Immun. 2016, 22, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.; Townsend, N.; Scarborough, P.; Rayner, M. Cardiovascular disease in Europe 2014: Epidemiological update. Eur. Heart J. 2014, 35, 2929. [Google Scholar] [CrossRef] [PubMed]
- Yeh, R.W.; Sidney, S.; Chandra, M.; Sorel, M.; Selby, J.V.; Go, A.S. Population trends in the incidence and outcomes of acute myocardial infarction. N. Engl. J. Med. 2010, 362, 2155–2165. [Google Scholar] [CrossRef] [PubMed]
- Facchin, B.M.; Dos Reis, G.O.; Vieira, G.N.; Mohr ET, B.; da Rosa, J.S.; Kretzer, I.F.; Demarchi, I.G.; Dalmarco, E.M. Inflammatory biomarkers on an LPS-induced RAW 264.7 cell model: A systematic review and meta-analysis. Inflamm. Res. 2022, 71, 741–758. [Google Scholar] [CrossRef]
- Li, X.; Peng, H.; Wu, J.; Xu, Y. Brain Natriuretic Peptide-Regulated Expression of Inflammatory Cytokines in Lipopolysaccharide (LPS)-Activated Macrophages via NF-kappaB and Mitogen Activated Protein Kinase (MAPK) Pathways. Med. Sci. Monit. 2018, 24, 3119–3126. [Google Scholar] [CrossRef]
- Song, Z.; Cui, Y.; Ding, M.-Z.; Jin, H.-X.; Gao, Y. Protective effects of recombinant human brain natriuretic peptide against LPS-Induced acute lung injury in dogs. Int. Immunopharmacol. 2013, 17, 508–512. [Google Scholar] [CrossRef]
- Chen, Z.; Hong, L.; Wang, H.; Yin, Q. Clinical study of recombinant human brain natriuretic peptide in patients with acute myocardial infarction complicating congestive heart failure. Heart 2011, 97, A149–A150. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Izzi, V.; D’Aquilio, F.; Carotenuto, F.; Di Nardo, P.; Baldini, P. Brain Natriuretic Peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul. Pept. 2008, 148, 26–32. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Vollmar, A.M. The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann. Rheum. Dis. 2001, 60 (Suppl. 3), iii68–iii70. [Google Scholar]
- Kiemer, A.K.; Hartung, T.; Vollmar, A.M. cGMP-mediated inhibition of TNF-α production by the atrial natriuretic peptide in murine macrophages. J. Immunol. 2000, 165, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhuo, N.; Zhang, J.; Sun, Q.; Si, J.; Wang, K.; Zhi, D. The loading of C-type natriuretic peptides improved hemocompatibility and vascular regeneration of electrospun poly(epsilon-caprolactone) grafts. Acta Biomater. 2022, 151, 304–316. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.M.; Spiller, K.L. Pro-inflammatory polarization primes Macrophages to transition into a distinct M2-like phenotype in response to IL-4. J. Leukoc. Biol. 2022, 111, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Ma, Y.; Iyer, R.P.; DeLeon-Pennell, K.Y.; Yabluchanskiy, A.; Garrett, M.R.; Lindsey, M.L. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res. Cardiol. 2017, 112, 1–14. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Rajasingh, J.; Lambers, E.; Qin, G.; Losordo, D.W.; Kishore, R. IL-10 inhibits inflammation and attenuates left ventricular remodeling after myocardial infarction via activation of STAT3 and suppression of HuR. Circ. Res. 2009, 104, e9–e18. [Google Scholar] [CrossRef]
- Yang, Z.; Zingarelli, B.; Szabó, C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation 2000, 101, 1019–1026. [Google Scholar] [CrossRef]
- Dominguez Rodriguez, A.; Abreu Gonzalez, P.; Garcia Gonzalez, M.J.; Ferrer Hita, J. Association between serum interleukin 10 level and development of heart failure in acute myocardial infarction patients treated by primary angioplasty. Rev. Esp. Cardiol. 2005, 58, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Kuhn, R.; Rajewsky, K.; Muller, W.; Menon, S.; Davidson, N.; Grunig, G.; Rennick, D. Interleukin-10 is a central regulator of the response to LPS in murine models of endotoxic shock and the Shwartzman reaction but not endotoxin tolerance. J. Clin. Investig. 1995, 96, 2339–2347. [Google Scholar] [CrossRef]
- Ocuin, L.M.; Bamboat, Z.M.; Balachandran, V.P.; Cavnar, M.J.; Obaid, H.; Plitas, G.; DeMatteo, R.P. Neutrophil IL-10 suppresses peritoneal inflammatory monocytes during polymicrobial sepsis. J. Leukoc. Biol. 2011, 89, 423–432. [Google Scholar] [CrossRef]
- Perretti, M.; Szabo, C.; Thiemermann, C. Effect of interleukin-4 and interleukin-10 on leucocyte migration and nitric oxide production in the mouse. Br. J. Pharmacol. 1995, 116, 2251–2257. [Google Scholar] [CrossRef]
- Weston, L.L.; Jiang, S.; Chisholm, D.; Jantzie, L.L.; Bhaskar, K. Interleukin-10 deficiency exacerbates inflammation-induced tau pathology. J. Neuroinflammation 2021, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Tesoro, L.; Hernandez, I.; Ramirez-Carracedo, R.; Diez-Mata, J.; Alcharani, N.; Jimenez-Guirado, B.; Ovejero-Paredes, K.; Filice, M.; Zamorano, J.L.; Saura, M.; et al. NIL10: A New IL10-Receptor Binding Nanoparticle That Induces Cardiac Protection in Mice and Pigs Subjected to Acute Myocardial Infarction through STAT3/NF-kappaB Activation. Pharmaceutics 2022, 14, 2044. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the MTT Assay. Cold Spring Harb. Protoc. 2018, 2018, pdb-prot095505. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhou, M.; Liu, Z.; Chen, Y.; Li, R. Inhibition effects of low concentrations of epigallocatechin gallate on the biofilm formation and hemolytic activity of Listeria monocytogenes. Food Control. 2018, 85, 119–126. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzazi, D.; Mami, W.; Mosbah, A.; Marrakchi, N.; Ben Ahmed, M.; Messadi, E. Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner. Toxins 2023, 15, 298. https://doi.org/10.3390/toxins15040298
Bouzazi D, Mami W, Mosbah A, Marrakchi N, Ben Ahmed M, Messadi E. Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner. Toxins. 2023; 15(4):298. https://doi.org/10.3390/toxins15040298
Chicago/Turabian StyleBouzazi, Dorsaf, Wael Mami, Amor Mosbah, Naziha Marrakchi, Melika Ben Ahmed, and Erij Messadi. 2023. "Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner" Toxins 15, no. 4: 298. https://doi.org/10.3390/toxins15040298
APA StyleBouzazi, D., Mami, W., Mosbah, A., Marrakchi, N., Ben Ahmed, M., & Messadi, E. (2023). Natriuretic-like Peptide Lebetin 2 Mediates M2 Macrophage Polarization in LPS-Activated RAW264.7 Cells in an IL-10-Dependent Manner. Toxins, 15(4), 298. https://doi.org/10.3390/toxins15040298