Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microcystins Did Not Have an Effect on the Phospholipid Phase State, as Revealed by the Fluorescent Probe Laurdan
2.2. No Resonance Energy Transfer between MC-LW and the Fluorescent Probe Cholestatrienol
2.3. Dramatic Morphological Effects Caused by MC-LF and MC-LW

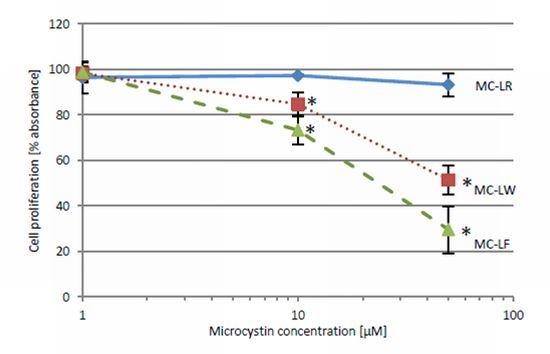
2.4. MC-LF and MC-LW Suppress Caco-2 Cell Proliferation
 standard deviation. Values marked with * are statistically different from control cells (Student’s t-Test, p ≤ 0.05).
standard deviation. Values marked with * are statistically different from control cells (Student’s t-Test, p ≤ 0.05).
 standard deviation. Values marked with * are statistically different from control cells (Student’s t-Test, p ≤ 0.05).
standard deviation. Values marked with * are statistically different from control cells (Student’s t-Test, p ≤ 0.05).
2.5. MC-LF and MC-LW Induced Caco-2 Cell Death

2.6. MC-LF and MC-LW Were Weaker Protein Phosphatase Inhibitors

3. Experimental Section
3.1. Reagents
3.2. Generalized Polarization

3.3. Fluorescence Resonance Energy Transfer
3.4. Caco-2 Cells
3.5. Cell Morphology
3.6. Cell Proliferation
3.7. Cytotoxicity Assay
3.8. Protein Phosphatase Inhibition
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Codd, G.A.; Morrison, L.F.; Metcalf, J.S. Cyanobacterial toxins: Risk management for health protection. Toxicol. Appl. Pharmacol. 2005, 203, 264–272. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Cyanobacterial Toxins. In Toxic Cyanobacteria in Water: A guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; E & FN Spon: London, UK, 1999; pp. 41–111. [Google Scholar]
- Del Campo, F.F.; Ouahid, Y. Identification of microcystins from three collection strains of microcystis aeruginosa. Environ. Pollut. 2010, 158, 2906–2914. [Google Scholar] [CrossRef]
- Sano, T.; Takagi, H.; Kaya, K. A dhb-microcystin from the filamentous cyanobacterium planktothrix rubescens. Phytochemistry 2004, 65, 2159–2162. [Google Scholar] [CrossRef]
- Botes, D.P.; Tuinman, A.A.; Wessels, P.L.; Viljoen, C.C.; Kruger, H.; Williams, D.H.; Santikarn, S.; Smith, R.J.; Hammond, S.J. The structure of cyanoginosin-la, a cyclic heptapeptide toxin from the cyanobacterium microcystis aeruginosa. J. Chem. Soc. Perkin Trans. 1984. [Google Scholar]
- Botes, D.P.; Wessels, L.; Kruger, H.; Runnegar, M.T.C.; Santikarn, S.; Smith, R.J.; Barna, J.C.J.; Williams, D.M. Structural studies on cyanoginosins-lr,-yr,-ya, and-ym, peptide toxins from microcystis aeruginosa. J. Chem. Soc. Perkin Trans. 1985. [Google Scholar]
- Rinehart, K.L.; Harada, K.; Namikoshi, M.; Chen, C.; Harvis, C.A.; Munro, M.H.G.; Blunt, J.W.; Mulligan, P.E.; Beasley, V.R.; Dahlem, A.M.; et al. Nodularin, microcystin, and the configuration of adda. J. Am. Chem. Soc. 1988, 110, 8557–8558. [Google Scholar]
- Rinehart, K.L.; Namikoshi, M.; Choi, B.W. Structure and biosynthesis of toxins from blue-green algae (cyanobacteria). J. Appl. Phycol. 1994, 6, 159–176. [Google Scholar] [CrossRef]
- World Health Organization, Guidelines for Drinking Water Quality, 4th edWorld Health Organization: Geneva, Switzerland, 2011.
- Gupta, N.; Pant, S.C.; Vijayaraghavan, R.; Rao, P.V. Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (lr, rr, yr) in mice. Toxicology 2003, 188, 285–296. [Google Scholar] [CrossRef]
- Monks, N.R.; Liu, S.; Xu, Y.; Yu, H.; Bendelow, A.S.; Moscow, J.A. Potent cytotoxicity of the phosphatase inhibitor microcystin lr and microcystin analogues in oatp1b1- and oatp1b3-expressing hela cells. Mol. Cancer Ther. 2007, 6, 587–598. [Google Scholar]
- Puerto, M.; Pichardo, S.; Jos, A.; Camean, A.M. Oxidative stress induced by microcystin-lr on plhc-1 fish cell line. Toxicol. in Vitro 2009, 23, 1445–1449. [Google Scholar]
- Ward, C.J.; Codd, G.A. Comparative toxicity of four microcystins of different hydrophobicities to the protozoan, tetrahymena pyriformis. J. Appl. Microbiol. 1999, 86, 874–882. [Google Scholar] [CrossRef]
- Gkelis, S.; Harjunpaa, V.; Lanaras, T.; Sivonen, K. Diversity of hepatotoxic microcystins and bioactive anabaenopeptins in cyanobacterial blooms from greek freshwaters. Environ. Toxicol. 2005, 20, 249–256. [Google Scholar]
- Spoof, L.; Vesterkvist, P.; Lindholm, T.; Meriluoto, J. Screening for cyanobacterial hepatotoxins, microcystins and nodularin in environmental water samples by reversed-phase liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogr. A 2003, 1020, 105–119. [Google Scholar] [CrossRef]
- Gurbuz, F.; Metcalf, J.S.; Karahan, A.G.; Codd, G.A. Analysis of dissolved microcystins in surface water samples from kovada lake, turkey. Sci. Total Environ. 2009, 407, 4038–4046. [Google Scholar] [CrossRef]
- Bittencourt-Oliveira, M.C.; Oliveira, M.C.; Pinto, E. Diversity of microcystin-producing genotypes in brazilian strains of microcystis (cyanobacteria). Braz. J. Biol. 2011, 71, 209–216. [Google Scholar] [CrossRef]
- Dietrich, D.; Hoeger, S. Guidance values for microcystins in water and cyanobacterial supplement products (blue-green algal supplements): A reasonable or misguided approach? Toxicol. Appl. Pharmacol. 2005, 203, 273–289. [Google Scholar] [CrossRef]
- Eriksson, J.E.; Toivola, D.; Meriluoto, J.A.; Karaki, H.; Han, Y.G.; Hartshorne, D. Hepatocyte deformation induced by cyanobacterial toxins reflects inhibition of protein phosphatases. Biochem. Biophys. Res. Commun. 1990, 173, 1347–1353. [Google Scholar] [CrossRef]
- MacKintosh, C.; Beattie, K.A.; Klumpp, S.; Cohen, P.; Codd, G.A. Cyanobacterial microcystin-lr is a potent and specific inhibitor of protein phosphatases 1 and 2a from both mammals and higher plants. FEBS Lett. 1990, 264, 187–192. [Google Scholar] [CrossRef]
- MacKintosh, R.W.; Dalby, K.N.; Campbell, D.G.; Cohen, P.T.; Cohen, P.; MacKintosh, C. The cyanobacterial toxin microcystin binds covalently to cysteine-273 on protein phosphatase 1. FEBS Lett. 1995, 371, 236–240. [Google Scholar]
- Runnegar, M.; Berndt, N.; Kong, S.M.; Lee, E.Y.; Zhang, L. In vivo and in vitro binding of microcystin to protein phosphatases 1 and 2a. Biochem. Biophys. Res. Commun. 1995, 216, 162–169. [Google Scholar] [CrossRef]
- Yoshizawa, S.; Matsushima, R.; Watanabe, M.F.; Harada, K.; Ichihara, A.; Carmichael, W.W.; Fujiki, H. Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 1990, 116, 609–614. [Google Scholar] [CrossRef]
- Chen, T.; Cui, J.; Liang, Y.; Xin, X.; Young, D.O.; Chen, C.; Shen, P. Identification of human liver mitochondrial aldehyde dehydrogenase as a potential target for microcystin-lr. Toxicology 2006, 220, 71–80. [Google Scholar]
- Mikhailov, A.; Harmala-Brasken, A.S.; Hellman, J.; Meriluoto, J.; Eriksson, J.E. Identification of atp-synthase as a novel intracellular target for microcystin-lr. Chem. Biol. Interact. 2003, 142, 223–237. [Google Scholar] [CrossRef]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar]
- Zeller, P.; Clement, M.; Fessard, V. Similar uptake profiles of microcystin-lr and -rr in an in vitro human intestinal model. Toxicology 2011, 290, 7–13. [Google Scholar] [CrossRef]
- De Matos Alves Pinto, L.; Malheiros, S.V.P.; Lino, A.C.S.; de Paula, E.; Perillo, M.A. Hydroxyzine, promethazine and thioridazine interaction with phospholipid monomolecular layers at the air-water interface. Biophys. Chem. 2006, 119, 247–255. [Google Scholar]
- Lee, A.G. Biological membranes: The importance of molecular detail. Trends Biochem. Sci. 2011, 36, 493–500. [Google Scholar] [CrossRef]
- Vesterkvist, P.S.M.; Meriluoto, J.A.O. Interaction between microcystins of different hydrophobicities and lipid monolayers. Toxicon 2003, 41, 349–355. [Google Scholar] [CrossRef]
- Bagatolli, L.A. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006, 1758, 1541–1556. [Google Scholar] [CrossRef]
- Purdon, A.D.; Tinker, D.O.; Neumann, A.W. Detection of lipid phase transitions by surface tensiometry. Chem. Phys. Lipids 1976, 17, 344–352. [Google Scholar]
- Parasassi, T.; de Stasio, G.; d’Ubaldo, A.; Gratton, E. Phase fluctuation in phospholipid membranes revealed by laurdan fluorescence. Biophys. J. 1990, 57, 1179–1186. [Google Scholar]
- Parasassi, T.; de Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar]
- Clegg, R.M. Fluorescence resonance energy transfer. Curr. Opin. Biotechnol. 1995, 6, 103–110. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed; Kluvert Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Wu, P.G.; Brand, L. Resonance energy transfer: Methods and applications. Anal. Biochem. 1994, 218, 1–13. [Google Scholar] [CrossRef]
- Schroeder, F.; Nemecz, G.; Gratton, E.; Barenholz, Y.; Thompson, T.E. Fluorescence properties of cholestatrienol in phosphatidylcholine bilayer vesicles. Biophys. Chem. 1988, 32, 57–72. [Google Scholar] [CrossRef]
- Toivola, D.M.; Goldman, R.D.; Garrod, D.R.; Eriksson, J.E. Protein phosphatases maintain the organization and structural interactions of hepatic keratin intermediate filaments. J. Cell Sci. 1997, 110, 23–33. [Google Scholar]
- McDermott, C.M.; Nho, C.W.; Howard, W.; Holton, B. The cyanobacterial toxin, microcystin-lr, can induce apoptosis in a variety of cell types. Toxicon 1998, 36, 1981–1996. [Google Scholar] [CrossRef]
- Fladmark, K.E.; Brustugun, O.T.; Hovland, R.; Boe, R.; Gjertsen, B.T.; Zhivotovsky, B.; Doskeland, S.O. Ultrarapid caspase-3 dependent apoptosis induction by serine/threonine phosphatase inhibitors. Cell Death Differentiation 1999, 6, 1099–1108. [Google Scholar]
- Ding, W.X.; Shen, H.M.; Ong, C.N. Microcystic cyanobacteria extract induces cytoskeletal disruption and intracellular glutathione alteration in hepatocytes. Environ. Health Perspect. 2000, 108, 605–609. [Google Scholar]
- Gehringer, M.M. Microcystin-lr and okadaic acid-induced cellular effects: A dualistic response. FEBS Lett. 2004, 557, 1–8. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S.; El-Gewely, M.R. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 127–152. [Google Scholar]
- Korzeniewski, C.; Callewaert, D.M. An enzyme-release assay for natural cytotoxicity. J. Immunol. Methods 1983, 64, 313–320. [Google Scholar]
- Ufelmann, H.; Kruger, T.; Luckas, B.; Schrenk, D. Human and rat hepatocyte toxicity and protein phosphatase 1 and 2a inhibitory activity of naturally occurring desmethyl-microcystins and nodularins. Toxicology 2012, 293, 59–67. [Google Scholar] [CrossRef]
- Nobre, A.C.; Jorge, M.C.; Menezes, D.B.; Fonteles, M.C.; Monteiro, H.S. Effects of microcystin-lr in isolated perfused rat kidney. Braz. J. Med. Biol. Res. 1999, 32, 985–988. [Google Scholar]
- Milutinovic, A.; Zivin, M.; Zorc-Pleskovic, R.; Sedmak, B.; Suput, D. Nephrotoxic effects of chronic administration of microcystins -lr and -yr. Toxicon 2003, 42, 281–288. [Google Scholar] [CrossRef]
- Gaudin, J.; Huet, S.; Jarry, G.; Fessard, V. In vivo DNA damage induced by the cyanotoxin microcystin-lr: Comparison of intra-peritoneal and oral administrations by use of the comet assay. Mutat. Res. 2008, 652, 65–71. [Google Scholar] [CrossRef]
- Alverca, E.; Andrade, M.; Dias, E.; Bento, F.S.; Batoreu, M.C.; Jordan, P.; Silva, M.J.; Pereira, P. Morphological and ultrastructural effects of microcystin-lr from microcystis aeruginosa extract on a kidney cell line. Toxicon 2009, 54, 283–294. [Google Scholar] [CrossRef]
- Humpage, A.R.; Hardy, S.J.; Moore, E.J.; Froscio, S.M.; Falconer, I.R. Microcystins (cyanobacterial toxins) in drinking water enhance the growth of aberrant crypt foci in the mouse colon. J. Toxicol. Environ. Health Part A 2000, 61, 155–165. [Google Scholar] [CrossRef]
- Zegura, B.; Volcic, M.; Lah, T.T.; Filipic, M. Different sensitivities of human colon adenocarcinoma (caco-2), astrocytoma (ipddc-a2) and lymphoblastoid (ncnc) cell lines to microcystin-lr induced reactive oxygen species and DNA damage. Toxicon 2008, 52, 518–525. [Google Scholar] [CrossRef]
- Lankoff, A.; Carmichael, W.W.; Grasman, K.A.; Yuan, M. The uptake kinetics and immunotoxic effects of microcystin-lr in human and chicken peripheral blood lymphocytes in vitro. Toxicology 2004, 204, 23–40. [Google Scholar] [CrossRef]
- Qiu, T.; Xie, P.; Liu, Y.; Li, G.; Xiong, Q.; Hao, L.; Li, H. The profound effects of microcystin on cardiac antioxidant enzymes, mitochondrial function and cardiac toxicity in rat. Toxicology 2009, 257, 86–94. [Google Scholar]
- Chong, M.W.; Gu, K.D.; Lam, P.K.; Yang, M.; Fong, W.F. Study on the cytotoxicity of microcystin-lr on cultured cells. Chemosphere 2000, 41, 143–147. [Google Scholar] [CrossRef]
- Sicinska, P.; Bukowska, B.; Michalowicz, J.; Duda, W. Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-lr in vitro. Toxicon 2006, 47, 387–397. [Google Scholar] [CrossRef]
- Puerto, M.; Pichardo, S.; Jos, Á.; Prieto, A.I.; Sevilla, E.; Frías, J.E.; Cameán, A.M. Differential oxidative stress responses to pure microcystin-lr and microcystin-containing and non-containing cyanobacterial crude extracts on caco-2 cells. Toxicon 2010, 55, 514–522. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar]
- Ungell, A.-L.B. Caco-2 replace or refine? Drug Discov. Today 2004, 1, 423–430. [Google Scholar] [CrossRef]
- Behrens, I.; Kissel, T. Do cell culture conditions influence the carrier-mediated transport of peptides in caco-2 cell monolayers? Eur. J. Pharm. Sci. 2003, 19, 433–442. [Google Scholar] [CrossRef]
- Botha, N.; Gehringer, M.M.; Downing, T.G.; van de Venter, M.; Shephard, E.G. The role of microcystin-lr in the induction of apoptosis and oxidative stress in caco2 cells. Toxicon 2004, 43, 85–92. [Google Scholar]
- Fischer, A.; Hoeger, S.J.; Stemmer, K.; Feurstein, D.J.; Knobeloch, D.; Nussler, A.; Dietrich, D.R. The role of organic anion transporting polypeptides (oatps/slcos) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and oatp-transfected hek293 cells. Toxicol. Appl. Pharmacol. 2010, 245, 9–20. [Google Scholar] [CrossRef]
- Craig, M.; Luu, H.A.; McCready, T.L.; Williams, D.; Andersen, R.J.; Holmes, C.F. Molecular mechanisms underlying he interaction of motuporin and microcystins with type-1 and type-2a protein phosphatases. Biochem. Cell Biol. 1996, 74, 569–578. [Google Scholar] [CrossRef]
- Moorhead, G.; MacKintosh, R.W.; Morrice, N.; Gallagher, T.; MacKintosh, C. Purification of type 1 protein (serine/threonine) phosphatases by microcystin-sepharose affinity chromatography. FEBS Lett. 1994, 356, 46–50. [Google Scholar] [CrossRef]
- Goldberg, J.; Huang, H.B.; Kwon, Y.G.; Greengard, P.; Nairn, A.C.; Kuriyan, J. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 1995, 376, 745–753. [Google Scholar]
- Blom, J.F.; Juttner, F. High crustacean toxicity of microcystin congeners does not correlate with high protein phosphatase inhibitory activity. Toxicon 2005, 46, 465–470. [Google Scholar] [CrossRef]
- Meriluoto, J.; Lawton, L.; Harada, K. Isolation and detection of microcystins and nodularins, cyanobacterial peptide hepatotoxins. Methods Mol. Biol. 2000, 145, 65–87. [Google Scholar]
- Fischer, R.T.; Stephenson, F.A.; Shafiee, A.; Schroeder, F. [delta]5,7,9(11)-cholestatrien-3[beta]-ol: A fluorescent cholesterol analogue. Chem. Phys. Lipids 1984, 36, 1–14. [Google Scholar] [CrossRef]
- Björkbom, A.; Yamamoto, T.; Kaji, S.; Harada, S.; Katsumura, S.; Slotte, J.P. Importance of the phosphocholine linkage on sphingomyelin molecular properties and interactions with cholesterol; a study with phosphate oxygen modified sphingomyelin-analogues. Biochim. Biophys. Acta 2008, 1778, 1501–1507. [Google Scholar] [CrossRef]
- Hope, M.J.; Bally, M.B.; Webb, G.; Cullis, P.R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 1985, 812, 55–65. [Google Scholar] [CrossRef]
- Engelke, M.; Bojarski, P.; Bloss, R.; Diehl, H. Tamoxifen perturbs lipid bilayer order and permeability: Comparison of dsc, fluorescence anisotropy, laurdan generalized polarization and carboxyfluorescein leakage studies. Biophys. Chem. 2001, 90, 157–173. [Google Scholar] [CrossRef]
- An, J.; Carmichael, W.W. Use of a colorimetric protein phosphatase inhibition assay and enzyme linked immunosorbent assay for the study of microcystins and nodularins. Toxicon 1994, 32, 1495–1507. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vesterkvist, P.S.M.; Misiorek, J.O.; Spoof, L.E.M.; Toivola, D.M.; Meriluoto, J.A.O. Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells. Toxins 2012, 4, 1008-1023. https://doi.org/10.3390/toxins4111008
Vesterkvist PSM, Misiorek JO, Spoof LEM, Toivola DM, Meriluoto JAO. Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells. Toxins. 2012; 4(11):1008-1023. https://doi.org/10.3390/toxins4111008
Chicago/Turabian StyleVesterkvist, Pia S. M., Julia O. Misiorek, Lisa E. M. Spoof, Diana M. Toivola, and Jussi A. O. Meriluoto. 2012. "Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells" Toxins 4, no. 11: 1008-1023. https://doi.org/10.3390/toxins4111008
APA StyleVesterkvist, P. S. M., Misiorek, J. O., Spoof, L. E. M., Toivola, D. M., & Meriluoto, J. A. O. (2012). Comparative Cellular Toxicity of Hydrophilic and Hydrophobic Microcystins on Caco-2 Cells. Toxins, 4(11), 1008-1023. https://doi.org/10.3390/toxins4111008