Binding Affinity and Capacity for the Uremic Toxin Indoxyl Sulfate
Abstract
:1. Introduction
2. Results
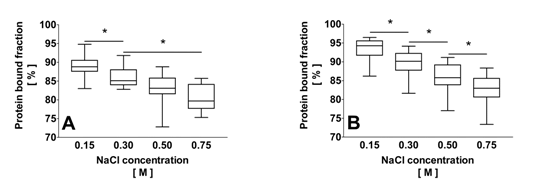
2.1. Binding of IS to Normal Human Plasma at Different Temperature and Ionic Strength


2.2. Correlation of the Ionic Strength with the Protein Bound Fraction of IS in Uremic Plasma

2.3. Effect of Dilution and Higher Ionic Strength on Toxin Binding in Human Plasma
| [NaCl] [M] | Dilution factor | KD [µM] | Bm [µM] | KD/Bm | Theoretically bound fraction α = 0.1 [%] | Experimentally bound fraction α = 0.1 [%] |
|---|---|---|---|---|---|---|
| 0.15 | 1:2 | 13.4 ± 3.6 | 261 ± 64 | 0.05 ± 0.02 | 94 ± 2 | 95 ± 2 (c) |
| 0.50 | 1:2 | 40.1 ± 18.4 | 297 ± 64 | 0.13 ± 0.05 (b) | 87 ± 4 (b) | 88 ± 4 (b) |
| 0.15 | 1:10 | 8.9 ± 2.7 | 59 ± 24 (a) | 0.16 ± 0.02 (a) | 85 ± 1 (a) | 86 ± 3 (a) |
| 0.50 | 1:10 | 44.7 ± 18.3 | 109 ± 27 (a) | 0.41 ± 0.11 (a,b) | 70 ± 5 (a,b) | 73 ± 6 (a,b) |
3. Discussion

4. Experimental Section
4.1. Modeling of the Protein Bound Toxin Fraction

4.2. Binding Capacity of Human Plasma at Different Temperature and Ionic Strength
4.3. Binding Affinity of Human Plasma for IS at Different NaCl Concentrations


4.4. Binding Capacity of Uremic Plasma for IS at Different NaCl Concentrations
4.5. Effect of Plasma Dilution on the Binding Capacity in Vitro
4.6. RP-HPLC Method
4.7. Determination of the Bound IS Fraction

4.8. Data Analysis
5. Conclusions
Abbreviations
| Bm | maximal binding capacity |
| ESRD | end-stage renal disease |
| IS | indoxyl sulfate |
| KA | association constant |
| KD | dissociation constant |
| NaCl | sodium chloride |
| RP-HPLC | reversed-phase high performance liquid chromatography |
Supplementary Files
Acknowledgments
Conflicts of Interest
References
- Niwa, T. Uremic toxicity of indoxyl sulfate. Nagoya J. Med. Sci. 2010, 72, 1–11. [Google Scholar]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef]
- Niwa, T.; Nomura, T.; Sugiyama, S.; Miyazaki, T.; Tsukushi, S.; Tsutsui, S. The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. Suppl. 1997, 62, S23–S28. [Google Scholar]
- Wu, I.W.; Hsu, K.H.; Lee, C.C.; Sun, C.Y.; Hsu, H.J.; Tsai, C.J.; Tsen, C.Y.; Wang, Y.C.; Lin, C.Y.; Wu, M.S. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol. Dial. Transplant. 2011, 26, 938–947. [Google Scholar] [CrossRef]
- Liabeuf, S.; Barreto, D.V.; Barreto, F.C.; Meert, N.; Glorieux, G.; Schepers, E.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Free p-cresylsulphate is a predictor of mortality in patients at different stages of chronic kidney disease. Nephrol. Dial. Transplant. 2010, 25, 1183–1191. [Google Scholar] [CrossRef]
- Otagiri, M. A molecular functional study on the interactions of drugs with plasma proteins. Drug Metab. Pharmacokinet. 2005, 20, 309–323. [Google Scholar] [CrossRef]
- Bhattacharya, A.A.; Curry, S.; Franks, N.P. Binding of the general anesthetics propofol and halothane to human serum albumin. J. Biol. Chem. 2000, 275, 38731–38738. [Google Scholar] [CrossRef]
- Takamura, N.; Maruyama, T.; Otagiri, M. Effects of uremic toxins and fatty acids on serum protein binding of furosemide: Possible mechanism of the binding defect in uremia. Clin. Chem. 1997, 43, 2274–2280. [Google Scholar]
- Jourde-Chiche, N.; Dou, L.; Cerini, C.; Dignat-George, F.; Vanholder, R.; Brunet, P. Protein-bound toxins—Update 2009. Semin. Dial. 2009, 22, 334–339. [Google Scholar] [CrossRef]
- Sakai, T.; Yamasaki, K.; Sako, T.; Kragh-Hansen, U.; Suenaga, A.; Otagiri, M. Interaction mechanism between IS, a typical uremic toxin bound to site II, and ligands bound to site I of human serum albumin. Pharm. Res. 2001, 18, 520–524. [Google Scholar] [CrossRef]
- Sakai, T.; Takadate, A.; Otagiri, M. Characterization of binding site of uremic toxins on human serum albumin. Biol. Pharm. Bull. 1995, 18, 1755–1761. [Google Scholar] [CrossRef]
- Vanholder, R.; Hoefliger, N.; de Smet, R.; Ringoir, S. Extraction of protein bound ligands from azotemic sera: Comparison of 12 deproteinization methods. Kidney Int. 1992, 41, 1707–1712. [Google Scholar] [CrossRef]
- Aoronov, P.A.; Luo, F.J.G.; Plummer, N.S.; Quan, Z.; Holmes, S.; Hostetter, T.H.; Meyer, T.W. Colonic contribution to uremic solutes. J. Am. Soc. Nephrol. 2011, 22, 1769–1776. [Google Scholar] [CrossRef]
- De Smet, R.; Dhondt, A.; Eloot, S.; Galli, F.; Waterloos, M.A.; Vanholder, R. Effect of the super-flux cellulose triacetate dialyser membrane on the removal of non-protein-bound and protein-bound ureamic solutes. Nephrol. Dial. Transplant. 2007, 22, 2006–2012. [Google Scholar] [CrossRef]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemofiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef]
- Lesaffer, G.; de Smet, R.; Lameire, N.; Dhondt, A.; Duym, P.; Vanholder, R. Intradialytic removal of protein-bound uraemic toxins role of solute characteristics and of dialyser membrane. Nephrol. Dial. Transplant. 2000, 15, 50–57. [Google Scholar]
- Liabeuf, S.; Drüeke, T.B.; Massy, Z.A. Protein-bound uremic toxins: New insight from clinical studies. Toxins 2011, 3, 911–919. [Google Scholar] [CrossRef]
- Meert, N.; Eloot, S.; Waterloos, M.A.; van Landschoot, M.; Dhondt, A.; Glorieux, G.; Ledebo, I.; Vanholder, R. Effective removal of protein-bound uraemic solutes by different convective strategies: A prospective trial. Nephrol. Dial. Transplant. 2009, 24, 562–570. [Google Scholar]
- Meijers, B.K.I.; de Loor, H.; Bammens, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. p-Cresyl sulfate and indoxyl sulfate in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1932–1938. [Google Scholar] [CrossRef]
- Sirich, T.L.; Luo, F.J.G.; Plummer, N.S.; Hostetter, T.H.; Meyer, T.W. Selectively increasing the clearance of protein-bound uremic solutes. Nephrol. Dial. Transplant. 2012, 27, 1574–1579. [Google Scholar] [CrossRef]
- Meyer, T.W.; Paettie, J.W.T.; Miller, J.D.; Dinh, D.C.; Recht, N.S.; Walther, J.L.; Hostetter, T.H. Increasing the clearance of protein-bound solutes by addition of a sorbent to the dialysate. J. Am. Soc. Nephrol. 2007, 18, 868–874. [Google Scholar] [CrossRef]
- Meyer, T.; Leeper, E.C.; Barlett, D.W.; Depner, T.A.; Zhao Lit, Y.; Robertson, C.R.; Hostetter, T.H. Increasing dialysate flow and dialyzer mass transfer area coefficient to increase the clearance of protein-bound solutes. J. Am. Soc. Nephrol. 2004, 15, 1927–1935. [Google Scholar] [CrossRef]
- Viaene, L.; Annaert, P.; de Loor, H.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- Mozar, A.; Louvet, L.; Godin, C.; Mentaverri, R.; Brazier, M.; Kamel, S.; Massy, Z.A. Indoxyl sulphate inhibits osteoclast differentiation and function. Nephrol. Dial. Transplant. 2012, 27, 2176–2181. [Google Scholar] [CrossRef]
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl sulfate induces skeletal resistance to parathyroid hormone in cultured osteoblastic cells. Kidney Int. 2007, 71, 738–743. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef]
- Klammt, S.; Wojak, H.J.; Mitzner, A.; Koball, S.; Rychly, J.; Reisinger, E.C.; Mitzner, S. Albumin-binding capacity (ABiC) is reduced in patients with chronic kidney disease along with an accumulation of protein-bound uraemic toxins. Nephrol. Dial. Transplant. 2012, 27, 2377–2383. [Google Scholar] [CrossRef]
- Urbani, A.; Lupisella, S.; Sirolli, V.; Bucci, S.; Amoroso, L.; Pavone, B.; Pieroni, L.; Sacchetta, P.; Bonomini, M. Proteomic analysis of proteinadsorption capacity of different haemodialysis membranes. Mol. Biosyst. 2012, 8, 1029–1039. [Google Scholar] [CrossRef]
- Vanholder, R.; de Smet, R.; Glorieux, G.; Argilès, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; de Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; de Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argilès, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Nishio, T.; Takamura, N.; Nishii, R.; Tokunaga, J.; Yoshimoto, M.; Kawai, K. Influences of haemodialysis on the binding sites of human serum albumin: Possibility of an efficacious administration plan using binding inhibition. Nephrol. Dial. Transplant. 2008, 23, 2304–2310. [Google Scholar] [CrossRef]
- Watanabe, H.; Noguchi, T.; Miyamoto, Y.; Kadowaki, D.; Kotani, S.; Nakajima, M.; Miyamura, S.; Ishima, Y.; Otagiri, M.; Maruyama, T. Interaction between two sulfate-conjugated uremic toxins, p-cresyl sulfate and indoxyl sulfate, during binding with human serum albumin. Drug Metab. Dispos. 2012, 40, 1423–1428. [Google Scholar] [CrossRef]
- Van der Vusse, G.J. Albumin as fatty acid transporter. Drug Metab. Pharmacokinet. 2009, 24, 300–307. [Google Scholar] [CrossRef]
- Banerjee, T.; Singh, S.K.; Kishore, N. Binding of naproxen and amitriptyline to bovine serum albumin: Biophysical aspects. J. Phys. Chem. B 2006, 110, 24147–24156. [Google Scholar] [CrossRef]
- Olsen, H.; Andersen, A.; Nordbo, A.; Kongsgaard, U.E.; Bormer, O.P. Pharmaceutical-grade albumin: Impaired drug-binding capacity in vitro. BMC Clin. Pharmacol. 2004, 4. [Google Scholar] [CrossRef] [Green Version]
- De Smet, R.; van Kaer, J.; van Vlem, B.; de Cubber, A.; Brunet, P.; Lameire, N.; Vanholder, R. Toxicity of free p-cresol: A prospective and cross-sectional analysis. Clin. Chem. 2003, 49, 470–478. [Google Scholar] [CrossRef]
- Weisiger, R.A.; Ostrow, J.D.; Koehler, R.K.; Webster, C.C.; Mukerjee, P.; Pascolo, L.; Tiribelli, C. Affinity of human serum albumin for bilirubin varies with albumin concentration and buffer composition. J. Biol. Chem. 2001, 276, 29953–29960. [Google Scholar]
- Bergé-Lefranc, D.; Chaspoul, F.; Calaf, R.; Charpiot, P.; Brunet, P.; Gallice, P. Binding of p-cresylsulfate and p-cresol to human serum albumin studied by microcalorimetry. J. Phys. Chem. B 2010, 114, 1661–1665. [Google Scholar] [CrossRef]
- Schwinn, D.A.; Schafer, S.L. Anesthesia; Miller, R.D., Ed.; Churchill Livingstone: London, UK, 2000; pp. 15–48. [Google Scholar]
- Meijers, B.K.I.; Bammens, B.; de Moor, B.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. Free p-cresol is associated with cardiovascular disease in hemodialysis patients. Kidney Int. 2008, 73, 1174–1180. [Google Scholar] [CrossRef]
- De Smet, R.; van Kaer, J.; Liebich, H.; Lesaffer, G.; Verstraete, A.; Dhondt, A.; Duym, P.; Lameire, N.; Vanholder, R. Heparin-induced release of protein-bound solutes during hemodialysis is an in vitro artifact. Clin. Chem. 2001, 47, 901–909. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Devine, E.; Krieter, D.H.; Rüth, M.; Jankovski, J.; Lemke, H.-D. Binding Affinity and Capacity for the Uremic Toxin Indoxyl Sulfate. Toxins 2014, 6, 416-429. https://doi.org/10.3390/toxins6020416
Devine E, Krieter DH, Rüth M, Jankovski J, Lemke H-D. Binding Affinity and Capacity for the Uremic Toxin Indoxyl Sulfate. Toxins. 2014; 6(2):416-429. https://doi.org/10.3390/toxins6020416
Chicago/Turabian StyleDevine, Eric, Detlef H. Krieter, Marieke Rüth, Joachim Jankovski, and Horst-Dieter Lemke. 2014. "Binding Affinity and Capacity for the Uremic Toxin Indoxyl Sulfate" Toxins 6, no. 2: 416-429. https://doi.org/10.3390/toxins6020416
APA StyleDevine, E., Krieter, D. H., Rüth, M., Jankovski, J., & Lemke, H. -D. (2014). Binding Affinity and Capacity for the Uremic Toxin Indoxyl Sulfate. Toxins, 6(2), 416-429. https://doi.org/10.3390/toxins6020416