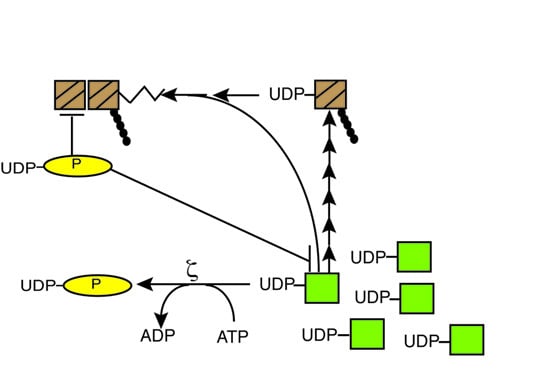
2.1. Toxin ζ Hydrolyses ATP in the Presence of UNAG
Recently, it was documented that in the presence of ATP-Mg
2+, ζ toxin, in the presence of ε
2 antitoxin, phosphorylates the cell wall precursor UNAG by attaching a phosphoryl group to the 3'-hydroxyl group of the
N-acetylglucosamine moiety to form UDP-
N-acetylglucosamine-3'-phosphate (UNAG-3P) [
9]. The low efficiency of this reaction, which was not stoichiometric, could be attributed to the presence of the antitoxin or because the phosphorylation reaction might be uncoupled from the ATPase reaction. In this work we have we tested whether ζ toxin purified by a novel protocol, free of antitoxin (as described in the
Experimental section), is able to hydrolyze ATP in the presence or absence of UNAG. We have also tested whether the hydrolysis reaction is coupled with the phosphotransfer reaction and if GTP could work as a donor of inorganic phosphate (P
i) for the transfer reaction. The products were separated from the substrate by thin-layer chromatography (TLC) and analyzed by autoradiography.
In the presence of UNAG, ζ toxin hydrolyzed [γ32P]-ATP, and we observed the accumulation of a radioactive product(s) which co-migrate with the front (data not shown). This was the expected position for the product of ATP hydrolysis, which is inorganic phosphate [32Pi]. We do not know how [γ32P]-UNAG-3P should migrate under the conditions used, but one hypothesis is that it co-migrates with the TLC front under the experimental conditions used. To address this, we perform experiments with other TLC eluents in order to separate [32Pi] from [32P]-UNAG-P, but we failed to have a significant separation of both of them under the conditions used (data not shown). Alternatively, the reaction is uncoupled and [γ32P]-UNAG-P accounts only to a minor fraction of the radiolabelled product.
To quantify the rate of ATP hydrolysis the experiments were performed using [α
32P]-ATP. If ζ hydrolyses [α
32P]-ATP it should be converted onto [α
32P]-ADP that runs faster than [α
32P]-ATP in a TLC. The commercial radioactive [α
32P]-ATP contains 6%–10% of [α
32P]-ADP [
17], hence this is our background level, and this was used in the TLC as an internal control that marked the respective running positions of substrates and products. In the absence of ζ toxin no [α
32P]-ATP hydrolysis was observed (
Figure 1, lane 10). Purified ζ toxin (0.5 µM) did not hydrolyze [α
32P]-ATP (0.5 mM) in the absence of UNAG, but ζ toxin hydrolyzed >85% of the ATP substrate upon addition of UNAG (2 mM), in a 60 min reaction (
Figure 1, lanes 1 and 2).
Figure 1.
Uridine diphosphate-N-acetylglucosamine (UNAG)-dependent ζ hydrolysis of ATP is poorly competed by GTP. Samples containing 2 mM UNAG and increasing concentrations of ATP (0.5, 5 and 10 mM (with a fixed concentration of α32P-ATP, 10 nM), lanes 2–4) or 2 mM UNAG and a fixed concentration of ATP (0.5 mM [α32P-ATP, 10 nM]) and increasing GTP concentrations (1.25, 2.5, 5, 7.5 and 10 mM, lanes 5–9) were incubated with 0.5 µM ζ toxin for 30 min at 30 °C in buffer B. ATP hydrolysis was analyzed by thin-layer chromatography (TLC) performed on polyethyleneimine-cellulose plates with 0.85 M KH2PO4 (pH 3.4) as the mobile phase, followed by autoradiography.
Figure 1.
Uridine diphosphate-N-acetylglucosamine (UNAG)-dependent ζ hydrolysis of ATP is poorly competed by GTP. Samples containing 2 mM UNAG and increasing concentrations of ATP (0.5, 5 and 10 mM (with a fixed concentration of α32P-ATP, 10 nM), lanes 2–4) or 2 mM UNAG and a fixed concentration of ATP (0.5 mM [α32P-ATP, 10 nM]) and increasing GTP concentrations (1.25, 2.5, 5, 7.5 and 10 mM, lanes 5–9) were incubated with 0.5 µM ζ toxin for 30 min at 30 °C in buffer B. ATP hydrolysis was analyzed by thin-layer chromatography (TLC) performed on polyethyleneimine-cellulose plates with 0.85 M KH2PO4 (pH 3.4) as the mobile phase, followed by autoradiography.
When saturating ATP concentrations were used, also a large fraction of the ATP was hydrolyzed (
Figure 1, lanes 3 and 4). At ATP:UNAG ratios of 2.5:1 or 5:1 ~70% and ~40% of radiolabelled [α
32P]-ATP was converted onto [α
32P]-ADP, respectively, in a 60 min reaction (
Figure 1, lanes 3 and 4). Since the conversion of ATP onto ADP was significantly higher than if each P
i generated was transferred to the 3'-OH group of the sugar moiety of UNAG, we tentatively assume that the reaction is not tightly coupled. Previously, it was shown that ζ toxin, in the presence of ATP-Mg
2+ and the ε
2 antitoxin, attaches a phosphoryl group to the 3'-hydroxyl group of the
N-acetylglucosamine moiety to form UDP-
N-acetylglucosamine-3'-phosphate (UNAG-3P) [
9]. Hence we assume that ζ toxin alone (free of ε
2 antitoxin) would also transfer the P
i to form UNAG-3P, but it remains to be shown whether the 3'-OH group of the sugar moiety of UNAG is the only target of the phosphorylation reaction.
To verify the structurally suggested preference for ATP
vs. GTP (as reported for other phosphotransferases) as substrate, we performed competition experiments. Here, ζ toxin was incubated with increasing ATP or GTP concentrations containing a fixed amount of ATP (0.5 mM ATP containing [α-
32P]-ATP 10 nM) and UNAG (2 mM). Toxin ζ can hydrolyze ATP with similar efficiency in the presence of a 2.5-fold excess of GTP (
Figure 1, lane 5). GTP:ATP ratios of 5:1 to 10:1, marginally decreased ζ-mediated [α
32P]-ATP hydrolysis (
Figure 1, lanes 6 and 7). At GTP:ATP ratios of 15:1 and 20.5:1, ~37 and ~46% of [α
32P]-ATP was not converted to [α
32P]-ADP, respectively (
Figure 1, lanes 8 and 9). It is likely therefore that: (I) ζ toxin is preferentially a UNAG-dependent ATPase; (II) the phosphotransfer reaction might not simply be a one-step coupled reaction; and (III) ζ toxin preferentially hydrolyzed ATP-Mg
2+ over GTP-Mg
2+ in the presence of UNAG.
2.2. Toxin ζ Phosphorylates a Fraction of UNAG In Vitro
In the previous section was shown that ζ requires the presence of UNAG to hydrolyze ATP, suggesting that UNAG or UNAG-3P might stimulate ζ-mediated ATP hydrolysis. To further analyze this, UNAG and ATP were incubated in the absence or presence ζ and the products of the reaction were analyzed by mass spectrometry as described in the
Experimental section.
In a mock reaction, lacking the ζ toxin, phosphorylated UNAG was not observed (compare
Figure 2A,B). In all conditions the nucleotide (or compound) and its Na-bound (e.g., M-H
+ [ATP, 506.06 peak], the M-2H
+-Na
+ [ATP-Na
2, 528.05 peak] or even M-3H
+-2Na
+ forms (due to the contribution of the buffer used) were detected (
Figure 2A). In the presence of ζ toxin, a massive degradation of the ATP pool (506.06 Da peak, expected 507.18 Da) with subsequent accumulation of ADP (426.06 Da peak, expected 427.20 Da) was observed. However, the proportion of UNAG (606.15 Da UNAG peak, expected 607.35 Da) converted into a 687.35 Da product (UNAG-3P) with a small peak at 686.12 Da (UNAG-3P-Na
2) was detectable but poor (
Figure 2). A parallel TLC analysis of this reaction revealed that ζ toxin hydrolyzed more than 95% of the ATP substrate (data not shown).
As revealed in
Figure 2B, in the presence of ζ toxin traces of the ATP pool and a reduced fraction of UNAG were detected by mass spectrometry, when compared to the absence of ζ toxin (
Figure 2A). Since traces of ATP and a significant fraction of UNAG remained after 30 min reaction, it was assumed, as done in the previous section (
Figure 1), that only a fraction of the P
i was transferred to UNAG to generate UNAG-3P (
Figure 2B). Alternatively, ζ toxin phosphorylates UNAG in a coupled reaction, but under the conditions used we are loosing a fraction of the accumulated inactive UNAG-3P product.
Figure 2.
ζ toxin phosphorylates in vitro a fraction of UNAG. Samples containing 2 mM UNAG and 0.5 mM ATP were incubated in the absence (A) or presence (B) of ζ toxin (0.5 µM) for 60 min at 30 °C in buffer B. The reaction products were analyzed by mass spectroscopy. The peaks corresponding to relevant products (ATP, ADP, UNAG and UNAG-3P) are indicated. The intensity (×104) was expressed in arbitrary units (a.u.). In panel B, the 675 to 740 m/z section is enlarged in the insert.
Figure 2.
ζ toxin phosphorylates in vitro a fraction of UNAG. Samples containing 2 mM UNAG and 0.5 mM ATP were incubated in the absence (A) or presence (B) of ζ toxin (0.5 µM) for 60 min at 30 °C in buffer B. The reaction products were analyzed by mass spectroscopy. The peaks corresponding to relevant products (ATP, ADP, UNAG and UNAG-3P) are indicated. The intensity (×104) was expressed in arbitrary units (a.u.). In panel B, the 675 to 740 m/z section is enlarged in the insert.
2.3. Toxin ζY83C Leaves Significant Amounts of UNAG In Vivo
In vitro assays showed that ζ is a poor kinase, phosphorylating only a fraction of the UNAG present. However, these assays might not extrapolate to the in vivo situation. It could be that in vivo there is(are) some unknown factor(s) that may stimulate the phosphotransfer reaction. To test whether ζ toxin reduces or depletes the UNAG pool in vivo, and by this way it reduces or blocks the biosynthetic pathway of peptidoglycans different types of antimicrobials, which act at different levels of the two stage process of cell wall biosynthesis, were used.
It has been established that the first step of peptidoglycan (murein) biosynthesis is a cytosolic stage that consists in the transfer of an enolpyruvate moiety from phosphoenolpyruvate (PEP) to the C3 position of
N-acetylglucosamine moiety of UNAG, a reaction catalyzed by two MurA enzymes in Firmicutes (the ubiquitous MurA [MurAA or MurA1] and MurAB [or MurA2] [
18]), to yield enolpyruvyl-UDP-
N-acetylglucosamine (EP-UNAG). The synthesis of these murein precursors can be inhibited by: (I) ζ-mediated phosphorylation of UNAG to form unreactive UNAG-3P; and (II) a naturally occurring PEP analogue, Fosfomycin (Fos), which binds covalently to the active site of MurAA (or MurAB). Fos, in the presence of the UNAG substrate inhibits cell wall biosynthesis by blocking the accumulation of EP-UNAG [
19,
20]. EP-UNAG is transformed by a series of cytosolic steps, which results in the formation of the UDP-
N-acetyl-muramic acid-pentapeptide that is translocated to the membrane acceptor and through different steps the
N-acetylglucosamine/
N-acetylmuramic acid-pentapeptide is covalently linked to the lipid carrier molecule undecaprenol via a pyrophosphate ester bridge (Lipid II). Vancomycin (Van) prevents incorporation of
N-acetylglucosamine and
N-acetylmuramic acid-peptapeptide subunits into the peptidoglycan matrix, and interferes with the Lipid II cycle [
21]. Then, the Lipid II building block is used as the substrate for the polymerization reaction, consisting of the assembly into glycan chains by transglycosilation and peptide cross-linking of the pentapeptide moiety by transpeptidation, reactions catalyzed by Classes A or B penicillin-binding proteins [
22,
23,
24]. Amp acts as an irreversible inhibitor of these transpeptidases [
25,
26].
We reasoned that if ζ toxin depletes the UNAG pool, cells will become tolerant to antimicrobials that act downstream of toxin action, leading to non-inheritable drug tolerance (persistence). To test this hypothesis, ζ toxin induced cells were exposed to Fos, Van or Amp and the antimicrobial drug tolerance was analyzed. These antimicrobials, in addition of inhibiting cell wall synthesis as explained above, induce two regulatory systems to cope with stress in Firmicutes: the extracytoplasmic function (ECF) σ factors and/or cell envelope stress-sensing two component systems, which are linked to other stress responses [
27]. Van, which is the strongest cell envelope-perturbing agent, induces the synthesis of a large number of genes controlled by specific σ factors (σ
V, σ
M, σ
W and σ
Y) and two components systems (e.g., LiaRS), Fos alters the expression of genes controlled by σ
M and σ
W; and Amp alters the expression of genes through cell envelope stress-sensing two component systems (e.g., BlaRI, MecRI) [
27]. If the three antimicrobials show a similar behavior upon ζ toxin we can omit any specific contribution of cell envelop stress.
To perform these experiments we took advantage of the short-lived toxin variant, ζY83C (half-life ~28 min) under control of a Xyl inducible promoter (
xylR PXylAζY83C expression cassette) (
Table 1). The level of toxin in non-induced BG689 cells is too low (<10 ζY83C monomers/colony forming units, CFUs) to measurably alter the growth rate in minimal medium S7 (MMS7) [
12]. Previously it was observed that induction of the
xylR PXylAζY83C cassette (BG689 cells), by addition of 0.5% Xyl, increased ζY83C levels to a plateau with a toxin concentration of ~300 toxin monomers/CFUs at ~10 min, and the steady-state level of the toxin remained for at least 240 min [
12]. When exponentially growing BG689 cells, growing in MMS7, reached moderate-density (~5 × 10
7 cells/mL) xylose (Xyl) 0.5% was added to express ζY83C toxin as described Materials and Methods [
14,
15]. As already observed, transient exposure to sub-physiological concentrations of free ζY83C induced dormancy and produced a typical biphasic survival curve, with a sub-fraction of cells (1–5 × 10
−5 cells) non-inheritable tolerant to toxin action upon 120 min of exposure (
Figure 3) [
14,
15].
Table 1.
Bacterial strains used.
Table 1.
Bacterial strains used.
| Strains | Relevant genotype | Reference |
|---|
| BG687 a | +xylR, PxylA, cat | [12] |
| BG689 a | +xylR, PxylA ζY83C, cat | [12] |
| BG1127 a | +lacI, Phsp, spc, [pCB799, xylR, PxylA ε, cat] | [12] |
| BG1125 a,b | +lacI, Phsp ζ, spc, [pCB799, xylR, PxylA ε, cat] | [12] |
| BL21(DE3) c | +[pCB920, PT7 ζ gene, Pω ω and ε genes, bla] | This work |
When exponentially growing BG689 cells (~5 × 10
7 cells/mL in MMS7) were transiently exposed to Fos or Amp (at twice the minimal inhibitory concentration [MIC]) or to Van (at 4 times the MIC) for 120 min, these bactericidal antimicrobials caused a cessation of cell proliferation, and rendered a fraction of 0.8 to 5 × 10
−3 phenotypic tolerants (
Figure 3). This multidrug tolerance of the isogenic population of antimicrobial sensitive cells is termed bacterial persistence [
28,
29,
30].
To test whether toxin action depletes the UNAG pool and this leads to enhanced antimicrobial persistence when cells are treated with cell wall inhibitors moderate-density exponentially growing BG689 cells were treated with Xyl (to induce ζY83C toxin expression) and transiently exposed to the antimicrobial. Experiments are showed when the two compounds (
i.e., Xyl and the antimicrobial) were added simultaneously, but the same results were obtained when first cells were exposed to toxin action for 30 min, and later the antimicrobial was added. Toxin ζY83C expression and Fos, Van or Amp treatment additively decreased the rate of cell survivals to 0.9 to 2 × 10
−6 (
Figure 3). Since the three antimicrobial show a similar outcome in the presence of free ζY83C toxin, it is unlike that the enhanced sensitivity could be attributed to a perturbation of cell envelop stress (see above). It is likely that: (I) ζ toxin expression only decreases the UNAG pool, and Fos, Van or Amp use the remaining fraction to further inhibit the plating of toxin tolerant cells; and (II) ζ toxin expression additively enhances the efficacy of the antimicrobials rather than making cells insensitive to Fos, Van or Amp treatment. Alternatively, transient expression of ζ toxin, by inactivating the UNAG substrate, inhibits the action of the MurA-MurF enzymes, and this inhibition leads to loss of cell shape and integrity, with cells becoming more prone to autolysis, when they are additionally treated with any of these antimicrobial.
Figure 3.
Cell wall inhibitors and ζ toxin expression show an additive effect. BG689 cells containing the short living ζ variant (ζY83C) gene were grown to ~5 × 107 cell/mL in MMS7. Then Xyl (0.5% to induce expression of the ζY83C toxin) or an antimicrobial or both was added and the culture was incubated for 120 min. Cells were washed and appropriate dilutions were plated to count the survivals. The results are the average of at least four independent experiments and are within a 10% standard error.
Figure 3.
Cell wall inhibitors and ζ toxin expression show an additive effect. BG689 cells containing the short living ζ variant (ζY83C) gene were grown to ~5 × 107 cell/mL in MMS7. Then Xyl (0.5% to induce expression of the ζY83C toxin) or an antimicrobial or both was added and the culture was incubated for 120 min. Cells were washed and appropriate dilutions were plated to count the survivals. The results are the average of at least four independent experiments and are within a 10% standard error.
2.4. Prolonged Action of Toxin ζY83C Does not Induce Massive Cell Lysis
To test the latter hypothesis were analyzed if prolonged expression of sub-physiological concentrations of ζY83C (360 or 480 min) might exhaust the metabolite pool and BG689 cells might reach the limits of their “capacity” to exit the dormant state, and they cannot be longer rescued from the “point of no return”. As already observed, after 16 h of ζY83C toxin expression the survival rate drops to 2–8 × 10
−7 [
16].
BG689 cells were grown up to optical density (OD
600) of 0.2 in MMS7 and divided into two aliquots. To one aliquot inducer (0.5% Xyl) was added (time zero) and samples were collected at different times (120, 240, 360 and 480 min after induction) (
Figure 4). In the presence of Xyl, the increment in OD
600 halted after one doubling time, and remained constant during the 480 min interval (
Figure 4A). At these time points, samples were withdrawn, and the cells were stained with membrane-permeant SYTO 9 (green fluorescence) and membrane-impermeant PI (red fluorescence), appropriate dilutions were plated in LB plates and the survival rate was measured (
Figure 4B). Transient expression of ζY83C toxin for 120 min or longer periods of time induced dormancy, and a similar sub-fraction of 2–6 × 10
−5 survivals became tolerant of toxin action (
Figure 4B). Within the first 360 min of ζY83C toxin expression, the proportion of PI permeable cells slightly increased (~1.2-fold). At later times (480 min) the proportion of PI stained cells increased ~2-fold (~65% of total cells stained with PI) with respect to the proportion of cells stained with PI after 120 min of toxin expression (
Figure 4B). It is likely that prolonged ζY83C expression might not induce massive cell lysis.
Figure 4.
Effect of prolonged action of toxin ζY83C on membrane permeability and cell survival. (A) Growth curve of BG689 cells containing ζY83C gene. Cells were grown to ~2.5 × 107 cell/mL in MMS7 (OD600 = 0.2), to half of the culture Xyl (0.5%) was added to induce ζY83C expression (time zero) and OD600 was followed over time in the two cultures. The arrow denotes the time of Xyl (0.5%) addition; (B) Aliquots of the cultures were taken at time zero, 120, 360 and 480 min after Xyl addition (denoted as +). The cells were fixed, stained with SYTO 9 and PI, and analyzed by fluorescence microscopy (black lane), at the same time, cells were washed and appropriate dilutions were plated in LB plates to count the survivals. The numbers of relative CFUs (black bars) at the indicated times after ζY83C toxin expression are relative to the non-induced control at the same time (denoted as -) taken as 1 (grey bar). Error bars show 95% confidence intervals of more than three independent experiments.
Figure 4.
Effect of prolonged action of toxin ζY83C on membrane permeability and cell survival. (A) Growth curve of BG689 cells containing ζY83C gene. Cells were grown to ~2.5 × 107 cell/mL in MMS7 (OD600 = 0.2), to half of the culture Xyl (0.5%) was added to induce ζY83C expression (time zero) and OD600 was followed over time in the two cultures. The arrow denotes the time of Xyl (0.5%) addition; (B) Aliquots of the cultures were taken at time zero, 120, 360 and 480 min after Xyl addition (denoted as +). The cells were fixed, stained with SYTO 9 and PI, and analyzed by fluorescence microscopy (black lane), at the same time, cells were washed and appropriate dilutions were plated in LB plates to count the survivals. The numbers of relative CFUs (black bars) at the indicated times after ζY83C toxin expression are relative to the non-induced control at the same time (denoted as -) taken as 1 (grey bar). Error bars show 95% confidence intervals of more than three independent experiments.
When similar experiments were performed in
E. coli cells a different outcome is observed depending of the growth medium rather than the speed of growth [
9,
31]: In LB medium (doubling time ~28 min), 60 min after overexpression of PezTΔC242, cells underwent a massive death with few surviving cells showing an ovoid morphology [
9]; in supplemented M9 medium (doubling time ~33 min), ζ toxin over-expression induced dormancy, and 60 min after induction massive filamentation, with subsequent growth recovery at later times was observed [
31]. At present, the source of these discrepancies remains unknown.
2.5. Toxin ζ Reversibly Induces a Halt in Cell Proliferation
Previously, it was shown that transient exposure (120 min) to physiological concentrations of free wt ζ reversibly induces dormancy, produces a sub-fraction of membrane-compromised cells to be stained with PI (25%–35% of total cells), and a minor subpopulation of
B. subtilis cells become tolerant of toxin action. Subsequent expression of the ε
2 antitoxin facilitates the exit from the dormant state and a fraction of membrane compromised cells recover their alteration of the membrane potential [
14].
Toxin ζ expression inactivated a fraction of UNAG and Fos used the remaining fraction of unphosphorylated UNAG to further inhibit cell proliferation (see
Figure 3). If ζ toxin expression only reduces the UNAG pool and/or UNAG-P poisons the exit from the dormant state, expression of ε
2 antitoxin might lead to different outcomes in the presence of Fos: First, if ζ toxin expression and Fos addition deplete the UNAG pool, cells should not be recovered by antitoxin expression and cell wall biosynthesis can be rescued only after
de novo synthesis of UNAG or UNAG-3P has to be re-activated by an unknown pathway. Second, the concert action of both ζ toxin expression and Fos addition triggers cell lysis by unbalancing the control of peptidoglycan biosynthesis. Finally, if ζ toxin expression decreases a fraction of the UNAG pool, without depleting it, ε
2 antitoxin expression may reverse toxin action and the remaining UNAG is used to rescue cell proliferation. To discriminate between all these possible outcomes, cell survival and the proportion of PI stained cells were analyzed after ε
2 antitoxin expression.
When exponentially growing BG1125 cells (
Table 1) reached a moderate-density (~5 × 10
7 cells/mL in MMS7) 1 mM IPTG was added to induce ζ toxin expression. As already observed, expression of nearly physiological wt ζ toxin concentrations for 120 min induced dormancy, a fraction of the cell population (~30%) was stained with PI, and ~1 × 10
−5 survivals tolerant to toxin action were obtained (
Table 2). Subsequent expression of the ε
2 antitoxin, after 120 min of IPTG addition, by Xyl (0.5%) addition, led to exit of the dormant state with CFUs increasing ~5000-fold, and only a small fraction (~10%) of cells were stained with PI (
Table 2) [
14,
15]. Alternatively, resumption of cell growth might dilute the relative proportion of PI stained cells up to 10% of total cells.
In the absence of IPTG, addition of Fos to moderate-density exponentially growing BG1125 cells for 120 min reduced plating efficiency of the cells to levels similar to the ones observed in the BG689 background (~3 × 10
−3 survivals), and as expected for a bactericidal antimicrobial the proportion of cells permeable to PI increased (~70% of total cells) (
Table 2). Expression of the ε
2 antitoxin under these conditions did nor alter the observed outcome (~70% of total cells stained with PI, and ~3 × 10
−3 survivals). When moderate-density exponentially growing BG1125 cells were exposed to toxin (by addition of 1 mM IPTG) and Fos action, the toxin markedly enhanced the efficacy of Fos treatment, but no additive effect on membrane permeation to PI was observed (
Table 2). Then, transient expression of the ε
2 antitoxin led to partial exit of the dormant state, which increased >2000-fold CFUs, but it failed to decrease the proportion of PI permeable cells (
Table 2). It is likely that: (I) toxin expression enhances Fos sensitivity, and such effect does not seem to correlate with increased rate of cell lysis; (II) neither toxin expression nor Fos addition deplete the UNAG pool, because ε
2 antitoxin expression facilitates the exit of the dormant state of a large fraction of cells; and (III) unregulated levels of murein synthesis, by transient toxin expression and Fos addition, do not induce massive cell autolysis. We propose that ζ toxin and Fos decrease the UNAG pool, but when ε
2 antitoxin expression halts ζ toxin activity, by forming an inactive complex (ζε
2ζ), the remaining active fraction of UNAG is processed by MurA enzymes and cells exit the ζ-induced dormant state with resumption of growth as expected for a toxin that is bacteriostatic in nature.
Table 2.
Additive effect of ζ toxin on Fos action and ε2 reversion of ζ-induced dormancy.
Table 2.
Additive effect of ζ toxin on Fos action and ε2 reversion of ζ-induced dormancy.
| Conditions a | TA c | % PI stained cells d | CFUs e |
|---|
| - | ζ− ε(+) | 2.6 (1250) | 2.3 × 108 |
| +IPTG b | ζ+ ε(+) | 30 (1314) | 3.8 × 103 |
| +IPTG + Xyl b | ζ+ ε+ | 9.8 (1642) | 2.2 × 107 |
| +Fos | ζ− ε(+) | 73 (1055) | 6.7 × 105 |
| +Fos + Xyl b | ζ− ε+ | 70 (1432) | 6.6 × 105 |
| +IPTG b + Fos | ζ+ ε(+) | 75 (1247) | <1.0 × 102 |
| +IPTG + Fos + Xyl b | ζ+ ε+ | 68 (1560) | 2.3 × 105 |