Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of DON on Non-Proliferating Caco-2 Cells

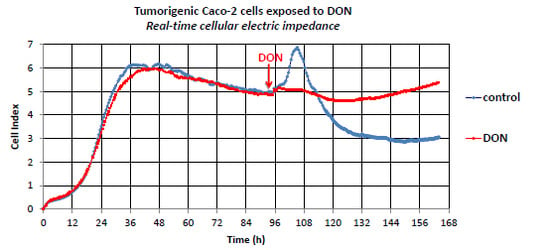
2.2. The Effect of DON on Actively Proliferating Caco-2 Cells


2.3. The Action of Lactobacillus Supernatant on DON-Treated Caco-2 Cells

3. Experimental Section
3.1. Mycotoxin
3.2. Lactobacillus Supernatant (LB Sup)
3.3. E. coli
3.4. Cells
3.5. Cell Cultures
3.6. Real-Time Cellular Impedance Measurements
3.7. The MTS Reduction Test
3.8. Statistic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Deoxynivalenol in food and feed: Occurrence and exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: poultry and swine. J. Appl. Toxicol. 2014. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”—Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Goyarts, T.; Danicke, S. Bioavailability of the Fusarium toxin deoxynivalenol (DON) from naturally contaminated wheat for the pig. Toxicol. Lett. 2006, 163, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrède, J.-P.; del Rio, J.-C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.-P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharm. 2009, 237, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.; Marin, D.; Bouhet, S.; Pinton, P.; Taranu, I.; Accensi, F. Immunotoxicological risk of mycotoxins for domestic animals. Food Addit. Contam. 2005, 22, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Goyarts, T.; Grove, N.; Danicke, S. Effects of the Fusarium toxin deoxynivalenol from naturally contaminated wheat given subchronically or as one single dose on the in vivo protein synthesis of peripheral blood lymphocytes and plasma proteins in the pig. Food Chem. Toxicol. 2006, 44, 1953–1965. [Google Scholar] [CrossRef] [PubMed]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, J.; Baines, J.; Bolger, M.; Duxbury, J.M.; Larsen, J.C.; Meyland, I.; Rao, M.V.; Renwick, A.G.; Schlatter, J.; Shephard, G.S.; et al. Evaluation of certain contaminants in food: Seventy-second report of the Joint FAO/WHO Expert Committee on Food Additives. In WHO Technical Report Series No. 959, 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Pinton, P.; Braicu, C.; Nougayrede, J.P.; Laffitte, J.; Taranu, I.; Oswald, I.P. Deoxynivalenol impairs porcine intestinal barrier function and decreases the protein expression of Claudin-4 through a mitogen-activated protein kinase-dependent mechanism. J. Nutr. 2010, 140, 1956–1962. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food. Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 1, 67–76. [Google Scholar] [CrossRef]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzoco, S. Nivalenol and Deoxynivalenol Affect Rat Intestinal Epithelial Cells: A Concentration Related Study. PLoS One 2012, 7, e52051. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, H.C.; Chen, S.J.; Liu, H.P.; Hsieh, Y.Y.; Yu, C.J.; Tang, R.; Hsieh, L.L.; Yu, J.S.; Chang, Y.S. Identification of collapsin response mediator protein-2 as a potential marker of colorectal carcinoma by comparative analysis of cancer cell secretomes. Proteomics 2008, 8, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Goossens, J.; Pasmans, F.; Verbrugghe, E.; Vandenbroucke, V.; de Baere, S.; Meyer, E.; Haesebrouck, F.; de Backer, P.; Croubels, S. Porcine intestinal epithelial barrier disruption by the Fusarium mycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Vet. Res. 2012, 8, 245–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, N.; Wang, X.; Xu, X.; Abassi, Y.A. The xCELLigence system for real-time and label-free monitoring of cell viability. Methods Mol. Biol. 2011, 740, 33–43. [Google Scholar] [PubMed]
- Diesing, A.K.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H.J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS One 2011, 6, e17472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Ren, H.J.; Li, X.G.; Sun, D.L.; Li, N.; Ming, L. Modulation of Intestinal Epithelial Cell Proliferation, Migration, and Differentiation in Vitro by Astragalus Polysaccharides. PLoS One 2014, 9, e106674. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-iphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lorenzo, A.; Rodriguez-Pineiro, A.M.; Rodriguez-Berrocal, F.J.; de la Cadena, M.P.; Martinez-Zorzano, V.S. Changes on the Caco-2 Secretome through Differentiation Analyzed by 2-D Differential In-Gel Electrophoresis (DIGE). Int. J. Mol. Sci. 2012, 13, 14401–14420. [Google Scholar] [CrossRef] [PubMed]
- Marin, D.E.; Taranu, I.; Motiu, M.; Manda, G. Effect of Lactobacillus feed supplement in deoxynivalenol intoxicated piglets. Arch. Zootech. 2010, 13, 12–22. [Google Scholar]
- Rosseli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc oxide protects cultured enterocytes from the damage induced by Escherichia coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manda, G.; Mocanu, M.A.; Marin, D.E.; Taranu, I. Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells. Toxins 2015, 7, 593-603. https://doi.org/10.3390/toxins7020593
Manda G, Mocanu MA, Marin DE, Taranu I. Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells. Toxins. 2015; 7(2):593-603. https://doi.org/10.3390/toxins7020593
Chicago/Turabian StyleManda, Gina, Mihaela Andreea Mocanu, Daniela Eliza Marin, and Ionelia Taranu. 2015. "Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells" Toxins 7, no. 2: 593-603. https://doi.org/10.3390/toxins7020593
APA StyleManda, G., Mocanu, M. A., Marin, D. E., & Taranu, I. (2015). Dual Effects Exerted in Vitro by Micromolar Concentrations of Deoxynivalenol on Undifferentiated Caco-2 Cells. Toxins, 7(2), 593-603. https://doi.org/10.3390/toxins7020593