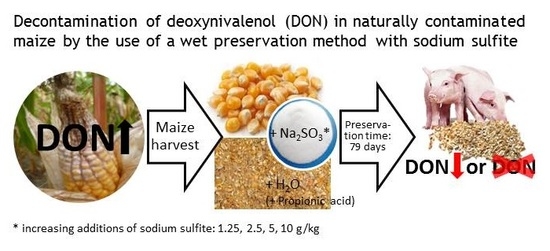
Effects of Increasing Concentrations of Sodium Sulfite on Deoxynivalenol and Deoxynivalenol Sulfonate Concentrations of Maize Kernels and Maize Meal Preserved at Various Moisture Content
Abstract
:1. Introduction
2. Results
2.1. Moisture Content and pH-Value
2.2. Deoxynivalenol Concentration
2.3. DON Sulfonate Concentrations
| Variant | Feed Matrix | Na2SO3 a (g/kg) | Measured Moisture (%) (n = 7) b | pH (Day 79) | Yeasts (Day 37, log CFU/g) | Yeasts (Day 79, log CFU/g) | Moulds (Day 37, log CFU/g) | Moulds (Day 79, log CFU/g) | DON Reduction (Day 79; %) | DONS 1 (Day 79, % of Initial DON) | DONS 2 (Day 79, % of Initial DON) | DONS 3 (Day 79, % of Initial DON) | Recovery (DON+DONS 1, 2 and 3, Day 79, % of Initial DON) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MK 1 | 0 | 14.6 ± 0.3 | 4.57 | n.d. | n.d. | 2.12 | n.d. | 7 | 0 | 0 | 0 | 93 |
| 2 | MK | 1.25 | 14.3 ± 0.5 | 4.76 | n.d. | n.d. | n.d. | n.d. | 41 | 0 | 24 | 8 | 91 |
| 3 | MK | 2.5 | 14.7 ± 0.6 | 4.80 | n.d. | 1.51 | n.d. | n.d. | 65 | 0 | 40 | 13 | 88 |
| 4 | MK | 5 | 14.7 ± 0.6 | 4.74 | n.d. | n.d. | n.d. | 1.81 | 79 | 0 | 54 | 19 | 94 |
| 5 | MK | 10 | 14.6 ± 0.7 | 4.87 | n.d. | n.d. | n.d. | n.d. | 87 | 0 | 48 | 18 | 79 |
| 6 | MK | 0 | 30.4 ± 0.6 | 4.63 | n.d. | n.d. | n.d. | 1.51 | 0 | 0 | 0 | 0 | 100 |
| 7 | MK | 1.25 | 30.5 ± 1.0 | 4.64 | n.d. | n.d. | n.d. | n.d. | 0 | 0 | 6 | 0 | 106 |
| 8 | MK | 2.5 | 31.1 ± 1.0 | 4.66 | n.d. | n.d. | n.d. | n.d. | 21 | 0 | 30 | 6 | 115 |
| 9 | MK | 5 | 31.2 ± 1.1 | 4.70 | n.d. | n.d. | n.d. | n.d. | 80 | 5 | 104 | 27 | 156 |
| 10 | MK | 10 | 30.0 ± 0.8 | 4.95 | n.d. | n.d. | n.d. | n.d. | 100 | 5 | 110 | 24 | 139 |
| 11 | MM 2 | 0 | 13.0 ± 0.2 | 4.55 | n.d. | 1.81 | 2.63 | 1.81 | 0 | 0 | 0 | 0 | 100 |
| 12 | MM | 1.25 | 13.0 ± 0.2 | 4.57 | n.d. | n.d. | n.d. | 1.51 | 4 | 0 | 1 | 0 | 97 |
| 13 | MM | 2.5 | 12.9 ± 0.2 | 4.61 | n.d. | n.d. | 2.0 | n.d. | 13 | 0 | 3 | 2 | 92 |
| 14 | MM | 5 | 13.3 ± 2.3 | 4.72 | n.d. | n.d. | n.d. | n.d. | 25 | 0 | 11 | 10 | 96 |
| 15 | MM | 10 | 13.0 ± 0.3 | 4.85 | n.d. | 1.51 | n.d. | n.d. | 40 | 0 | 21 | 21 | 102 |
| 16 | MM | 0 | 29.5 ± 0.4 | 4.56 | n.d. | n.d. | n.d. | n.d. | 0 | 0 | 0 | 0 | 100 |
| 17 | MM | 1.25 | 29.3 ± 0.5 | 4.59 | n.d. | 1.81 | n.d. | n.d. | 3 | 0 | 6 | 0 | 103 |
| 18 | MM | 2.5 | 29.3 ± 0.4 | 4.60 | n.d. | n.d. | n.d. | 1.51 | 45 | 2 | 46 | 9 | 112 |
| 19 | MM | 5 | 29.2 ± 0.3 | 4.71 | n.d. | n.d. | n.d. | n.d. | 91 | 5 | 93 | 18 | 125 |
| 20 | MM | 10 | 29.2 ± 0.3 | 4.86 | n.d. | n.d. | n.d. | n.d. | 100 | 6 | 113 | 18 | 137 |
| Moisture Content (%) | 14 | 30 | 14 | 30 |
|---|---|---|---|---|
| Feed Matrix | MK 1 | MK | MM 2 | MM |
| A | 22.88 | 100 | 3.43 | −57.96 |
| α | 1.7 | 89.77 | 0.08 | 67.62 |
| B | 23.8 | −49.12 | 48.13 | 109 |
| β1 | 0 | 496 | 13.07 | 30.14 |
| β2 | −808 | −1.03 | −1.29 | 305 |
| β3 | −2.72 | 0.32 | −0.16 | 0.001 |
| C | 20.37 | 61.77 | 50.54 | 55.57 |
| γ1 | 0.1 | 0.26 | 0.04 | 0.3 |
| γ2 | 2.21 | 9.57 | 10.73 | 7.74 |
| t1/2α (d) | 0.41 | 0.0077 | 9.05 | 0.0103 |
| Na2SO3 1/2γ1 (g Na2SO3/kg) | 7.06 | 2.69 | 15.41 | 2.35 |
| RSD (mg/kg) | 4.29 | 6.88 | 1.91 | 6.89 |
| r2 | 0.971 | 0.96 | 0.969 | 0.958 |
| Variants | Na2SO3 Addition | DONS Compound | Model | Regression Parameters ± SE | ymax | tmax (d) | t 1/2 AUC (d) | r2 | RSD | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Matrix + Moisture | DONS 1 | DONS 2 | DONS 3 | a | b | c | |||||||
| V9 | MK 1 30% | 5 | x | - | - | 1 | 2.46 ± 1.06 | 0.03 ± 0.02 | - | 2.26 | - | - | 0.941 | 0.30 |
| V10 | MK 30% | 10 | x | - | - | 1 | 2.16 ± 0.82 | 0.08 ± 0.04 | - | 2.16 | - | - | 0.946 | 0.30 |
| V18 | MM 2 30% | 2.5 | x | - | - | 1 | 1.26 ± 0.54 | 0.02 ± 0.01 | - | 0.96 | - | - | 0.978 | 0.09 |
| V19 | MM 30% | 5 | x | - | - | 1 | 2.47 ± 0.25 | 0.05 ± 0.01 | - | 2.42 | - | - | 0.980 | 0.19 |
| V20 | MM 30% | 10 | x | - | - | 1 | 2.86 ± 0.68 | 0.06 ± 0.03 | - | 2.83 | - | - | 0.876 | 0.51 |
| V2 | MK 14% | 1.25 | - | x | - | 1 | 14.01 ± 0.53 | 0.03 ± 0.002 | - | 12.53 | - | - | 0.999 | 0.27 |
| V3 | MK 14% | 2.5 | - | x | - | 1 | 20.81 ± 1.20 | 0.05 ± 0.01 | - | 20.28 | - | - | 0.992 | 1.02 |
| V4 | MK 14% | 5 | - | x | - | 1 | 28.61 ± 3.55 | 0.04 ± 0.01 | - | 26.85 | - | - | 0.978 | 2.14 |
| V5 | MK 14% | 10 | - | x | - | 1 | 24.28 ± 2.17 | 0.08 ± 0.02 | - | 24.24 | - | - | 0.971 | 2.37 |
| V7 | MK 30% | 1.25 | - | x | - | 1 | 5.50 ± 1.83 | 0.01 ± 0.004 | - | 2.91 | - | - | 0.993 | 0.14 |
| V8 | MK 30% | 2.5 | - | x | - | 1 | 16.83 ± 0.51 | 0.03 ± 0.002 | - | 15.36 | - | - | 0.999 | 0.29 |
| V9 | MK 30% | 5 | - | x | - | 1 | 89.06 ± 10.57 | 0.01 ± 0.002 | - | 53.66 | - | - | 0.998 | 1.15 |
| V10 | MK 30% | 10 | - | x | - | 1 | 75.41 ± 8.70 | 0.02 ± 0.003 | - | 56.43 | - | - | 0.996 | 1.94 |
| V12 | MM 14% | 1.25 | - | x | - | 1 | 0.74 ± 0.55 | 0.01 ± 0.02 | - | 0.49 | - | - | 0.907 | 0.09 |
| V13 | MM 14% | 2.5 | - | x | - | 1 | 39.55 ± 89.70 | 0.0005 ± 0.001 | - | 1.50 | - | - | 0.953 | 0.18 |
| V14 | MM 14% | 5 | - | x | - | 1 | 6.85 ± 1.35 | 0.02 ± 0.01 | - | 5.43 | - | - | 0.984 | 0.36 |
| V15 | MM 14% | 10 | - | x | - | 1 | 18.10 ± 8.83 | 0.01 ± 0.01 | - | 10.51 | - | - | 0.975 | 0.83 |
| V17 | MM 30% | 1.25 | - | x | - | 1 | 3.00 ± 0.33 | 0.07 ± 0.02 | - | 2.98 | - | - | 0.977 | 0.29 |
| V18 | MM 30% | 2.5 | - | x | - | 1 | 29.93 ± 3.25 | 0.02 ± 0.004 | - | 23.46 | - | - | 0.995 | 0.85 |
| V19 | MM 30% | 5 | - | x | - | 1 | 60.44 ± 3.40 | 0.02 ± 0.002 | - | 48.04 | - | - | 0.999 | 0.99 |
| V20 | MM 30% | 10 | - | x | - | 1 | 70.11 ± 7.46 | 0.02 ± 0.004 | - | 57.75 | - | - | 0.994 | 2.44 |
| V2 | MK 14% | 1.25 | - | - | x | 2 | 11.61 ± 0.22 | 0.14 ± 0.01 | 0.02 ± 0.001 | 13.13 | 6.52 | 38.59 | 0.998 | 0.38 |
| V3 | MK 14% | 2.5 | - | - | x | 2 | 27.91 ± 1.43 | 0.09 ± 0.02 | 0.03 ± 0.005 | 28.68 | 3.63 | 29.99 | 0.983 | 2.37 |
| V4 | MK 14% | 5 | - | - | x | 2 | 33.28 ± 1.58 | 0.04 ± 0.02 | 0.02 ± 0.004 | 32.66 | 1.64 | 32.55 | 0.984 | 2.58 |
| V5 | MK 14% | 10 | - | - | x | 2 | 39.65 ± 3.10 | 0.04 ± 0.03 | 0.03 ± 0.01 | 38.70 | 1.49 | 26.98 | 0.965 | 4.68 |
| V7 | MK 30% | 1.25 | - | - | x | 2 | 3.25 ± 0.24 | 0.04 ± 0.03 | 0.02 ± 0.01 | 3.20 | 1.93 | 31.94 | 0.962 | 0.43 |
| V8 | MK 30% | 2.5 | - | - | x | 2 | 20.20 ± 0.96 | 0.21 ± 0.03 | 0.04 ± 0.004 | 23.88 | 5.99 | 25.46 | 0.991 | 1.47 |
| V9 | MK 30% | 5 | - | - | x | 2 | 45.77 ± 0.71 | 0.07 ± 0.01 | 0.02 ± 0.001 | 47.07 | 3.96 | 40.51 | 0.998 | 1.27 |
| V10 | MK 30% | 10 | - | - | x | 2 | 50.21 ± 1.10 | 0.04 ± 0.01 | 0.02 ± 0.002 | 49.71 | 2.13 | 38.22 | 0.996 | 1.94 |
| V13 | MM 14% | 2.5 | - | - | x | 2 | 2.10 ± 0.15 | 0.01 ± 0.03 | 0.01 ± 0.004 | 2.09 | 1.50 | 90.53 | 0.949 | 0.28 |
| V14 | MM 14% | 5 | - | - | x | 2 | 5.95 ± 0.38 | 0.05 ± 0.03 | 0.004 ± 0.003 | 6.36 | 11.21 | 177.48 | 0.956 | 0.78 |
| V15 | MM 14% | 10 | - | - | x | 2 | 10.91 ± 0.47 | 0.00001 ± 0.02 | 0.0009 ± 0.002 | 10.91 | 0.01 | 783.60 | 0.977 | 0.98 |
| V17 | MM 30% | 1.25 | - | - | x | 2 | 3.75 ± 0.22 | 0.13 ± 0.03 | 0.03 ± 0.01 | 3.93 | 3.88 | 24.29 | 0.982 | 0.35 |
| V18 | MM 30% | 2.5 | - | - | x | 2 | 15.32 ± 1.23 | 0.26 ± 0.05 | 0.03 ± 0.005 | 21.20 | 9.52 | 34.77 | 0.984 | 0.98 |
| V19 | MM 30% | 5 | - | - | x | 2 | 35.65 ± 1.92 | 0.14 ± 0.03 | 0.02 ± 0.003 | 40.59 | 6.79 | 39.75 | 0.982 | 3.34 |
| V20 | MM 30% | 10 | - | - | x | 2 | 49.34 ± 0.54 | 0.05 ± 0.004 | 0.02 ± 0.001 | 48.87 | 2.24 | 33.06 | 0.999 | 0.93 |







3. Discussion
DON Sulfonate Concentrations
4. Experimental Section
4.1. Preservation Experiment
4.1.1. Contaminated Maize
4.1.2. Experimental Design
- Two different feed structures: maize kernels (MK) and maize grain meal (MM)
- Moisture contents of 14% and 30%
- Na2SO3 additions of 0, 1.25, 2.5, 5 and 10 g/kg maize
- Preservation duration of 0, 0.007 (10 min), 1, 3, 8, 16, 37 and 79 days.
| Variants | Feed Matrix | Planned Moisture Content (%) | Sodium Sulfite (g/kg Maize) |
|---|---|---|---|
| 1 | MK = Maize kernels | 14 | 0 |
| 2 | MK | 14 | 1.25 |
| 3 | MK | 14 | 2.5 |
| 4 | MK | 14 | 5 |
| 5 | MK | 14 | 10 |
| 6 | MK | 30 | 0 |
| 7 | MK | 30 | 1.25 |
| 8 | MK | 30 | 2.5 |
| 9 | MK | 30 | 5 |
| 10 | MK | 30 | 10 |
| 11 | MM = Maize meal | 14 | 0 |
| 12 | MM | 14 | 1.25 |
| 13 | MM | 14 | 2.5 |
| 14 | MM | 14 | 5 |
| 15 | MM | 14 | 10 |
| 16 | MM | 30 | 0 |
| 17 | MM | 30 | 1.25 |
| 18 | MM | 30 | 2.5 |
| 19 | MM | 30 | 5 |
| 20 | MM | 30 | 10 |
4.1.3. Procedures and Sample Preparation
4.2. Analyses
4.2.1. Mycotoxins
4.2.2. pH Value and Moisture Content
4.2.3. Microbiological Status
4.3. Statistics
- tmax = b/c (time at maximum concentration, d = days)
- ymax = α × tmaxb × ec × tmax (maximum concentration, mg/kg DM)
- t1/2AUC = gammainv (0.5, b+1, 1/c) (time which corresponds to the half of the AUC, h).
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rotter, B.A.; Prelusky, D.B.; Pestka, J.J. Toxicology of deoxynivalenol (vomitoxin). J. Toxicol. Environ. Health 1996, 48, 1–34. [Google Scholar]
- Döll, S.; Dänicke, S. The Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) in animal feeding. Prev. Vet. Med. 2011, 102, 132–145. [Google Scholar]
- Dorn, B.; Forrer, H.R.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Vogelgsang, S. Fusarium species complex and mycotoxins in grain maize from maize hybrid trials and from grower’s fields. J. Appl. Microbiol. 2011, 111, 693–706. [Google Scholar]
- Dänicke, S.; Valenta, H.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004, 58, 169–180. [Google Scholar]
- Goyarts, T.; Grove, N.; Dänicke, S. Effects of the Fusarium toxin deoxynivalenol from naturally contaminated wheat given subchronically or as one single dose on the in vivo protein synthesis of peripheral blood lymphocytes and plasma proteins in the pig. Food Chem. Toxicol. 2006, 44, 1953–1965. [Google Scholar]
- Young, J.C. Formation of sodium bisulfite addition-products with trichothecenones and alkaline-hydrolysis of deoxynivalenol and its sulfonate. J. Agric. Food Chem. 1986, 34, 919–923. [Google Scholar]
- Young, J.C.; Subryan, L.M.; Potts, D.; Mclaren, M.E.; Gobran, F.H. Reduction in levels of deoxynivalenol in contaminated wheat by chemical and physical treatment. J. Agric. Food Chem. 1986, 34, 461–465. [Google Scholar]
- Young, J.C.; Trenholm, H.L.; Friend, D.W.; Prelusky, D.B. Detoxification of deoxynivalenol with sodium bisulfite and evaluation of the effects when pure mycotoxin or contaminated corn was treated and given to pigs. J. Agric. Food Chem. 1987, 35, 259–261. [Google Scholar]
- Dänicke, S.; Valenta, H.; Gareis, M.; Lucht, H.W.; von Reichenbach, H. On the effects of a hydrothermal treatment of deoxynivalenol (DON)-contaminated wheat in the presence of sodium metabisulphite (Na2S2O5) on DON reduction and on piglet performance. Anim. Feed Sci. Technol. 2005, 118, 93–108. [Google Scholar]
- Dänicke, S.; Pahlow, G.; Goyarts, T.; Rohweder, D.; Wilkerling, K.; Breves, G.; Valenta, H.; Doll, S. Effects of increasing concentrations of sodium metabisulphite (Na2S2O5, SBS) on deoxynivalenol (DON) concentration and microbial spoilage of triticale kernels preserved without and with propionic acid at various moisture contents. Mycotoxin Res. 2009, 25, 215–223. [Google Scholar]
- Schwartz, H.E.; Hametner, C.; Slavik, V.; Greitbauer, O.; Bichl, G.; Kunz-Vekiru, E.; Schatzmayr, D.; Berthiller, F. Characterization of three deoxynivalenol sulfonates formed by reaction of deoxynivalenol with sulfur reagents. J. Agric. Food Chem. 2013, 61, 8941–8948. [Google Scholar]
- Schwartz-Zimmermann, H.E.; Wiesenberger, G.; Unbekannt, C.; Hessenberger, S.; Schatzmayr, D.; Berthiller, F. Reaction of (conjugated) deoxynivalenol with sulphur reagents—Novel metabolites, toxicity and application. World Mycotoxin J. 2014, 7, 187–197. [Google Scholar]
- European Commission. Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- Koehler, B. Natural mode of entrance of funghi into corn ears and some symptoms that indicate infection. J. Agric. Res. 1942, 64, 421–442. [Google Scholar]
- Young, J.C.; Fulcher, R.G.; Hayhoe, J.H.; Scott, P.M.; Dexter, J.E. Effect of milling and baking on deoxynivalenol (vomitoxin) content of eastern Canadian wheats. J. Agric. Food Chem. 1984, 32, 659–664. [Google Scholar]
- Beyer, M.; Danicke, S.; Rohweder, D.; Humpf, H.U. Determination of deoxynivalenol-sulfonate (DONS) in cereals by hydrophilic interaction chromatography coupled to tandem mass spectrometry. Mycotoxin Res. 2010, 26, 109–117. [Google Scholar]
- Schwartz-Zimmermann, H.E.; Paulick, M.; Danicke, S.; Schatzmayr, D.; Berthiller, F. Determination of deoxynivalenol sulphonates in cereal samples: Method development, validation and application. World Mycotoxin J. 2014, 7, 233–245. [Google Scholar]
- Dänicke, S.; Dusel, G.; Jeroch, H.; Kluge, H. Factors affecting efficiency of NSP-degrading enzymes in rations for pigs and poultry. Agribiol. Res. 1999, 52, 1–24. [Google Scholar]
- Dänicke, S.; Hegewald, A.K.; Kahlert, S.; Kluess, J.; Rothkotter, H.J.; Breves, G.; Döll, S. Studies on the toxicity of deoxynivalenol (DON), sodium metabisulfite, DON-sulfonate (DONS) and de-epoxy-DON for porcine peripheral blood mononuclear cells and the Intestinal Porcine Epithelial Cell lines IPEC-1 and IPEC-J2, and on effects of DON and DONS on piglets. Food Chem. Toxicol. 2010, 48, 2154–2162. [Google Scholar]
- Rempe, I.; Kersten, S.; Valenta, H.; Danicke, S. Hydrothermal treatment of naturally contaminated maize in the presence of sodium metabisulfite, methylamine and calcium hydroxide; effects on the concentration of zearalenone and deoxynivalenol. Mycotoxin Res. 2013, 29, 169–175. [Google Scholar]
- Naumann, C.; Bassler, R. Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 1993. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulick, M.; Rempe, I.; Kersten, S.; Schatzmayr, D.; Schwartz-Zimmermann, H.E.; Dänicke, S. Effects of Increasing Concentrations of Sodium Sulfite on Deoxynivalenol and Deoxynivalenol Sulfonate Concentrations of Maize Kernels and Maize Meal Preserved at Various Moisture Content. Toxins 2015, 7, 791-811. https://doi.org/10.3390/toxins7030791
Paulick M, Rempe I, Kersten S, Schatzmayr D, Schwartz-Zimmermann HE, Dänicke S. Effects of Increasing Concentrations of Sodium Sulfite on Deoxynivalenol and Deoxynivalenol Sulfonate Concentrations of Maize Kernels and Maize Meal Preserved at Various Moisture Content. Toxins. 2015; 7(3):791-811. https://doi.org/10.3390/toxins7030791
Chicago/Turabian StylePaulick, Marleen, Inga Rempe, Susanne Kersten, Dian Schatzmayr, Heidi Elisabeth Schwartz-Zimmermann, and Sven Dänicke. 2015. "Effects of Increasing Concentrations of Sodium Sulfite on Deoxynivalenol and Deoxynivalenol Sulfonate Concentrations of Maize Kernels and Maize Meal Preserved at Various Moisture Content" Toxins 7, no. 3: 791-811. https://doi.org/10.3390/toxins7030791
APA StylePaulick, M., Rempe, I., Kersten, S., Schatzmayr, D., Schwartz-Zimmermann, H. E., & Dänicke, S. (2015). Effects of Increasing Concentrations of Sodium Sulfite on Deoxynivalenol and Deoxynivalenol Sulfonate Concentrations of Maize Kernels and Maize Meal Preserved at Various Moisture Content. Toxins, 7(3), 791-811. https://doi.org/10.3390/toxins7030791