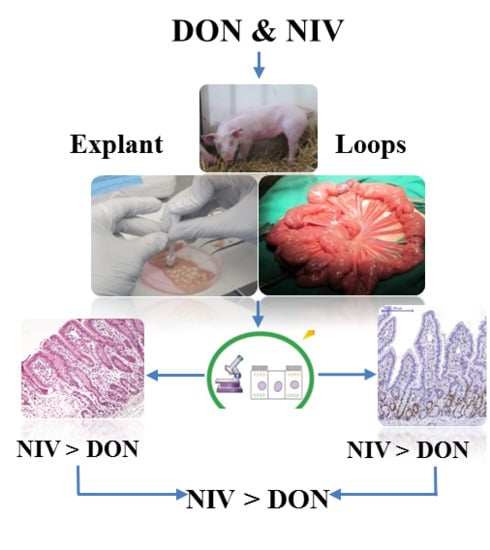
Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops
Abstract
:1. Introduction
2. Results
2.1. Explants Model
2.1.1. Histological Analysis before and after Incubation and Effect of DMSO

2.1.2. Effect of Mycotoxins on the Histological Scores

2.2. Loops Model
2.2.1. Comparison of Loops Segments with Non-Loops Segments
2.2.2. Effect of DON and NIV Exposure on Morphometry in the Loops

2.2.3. Effect of DON and NIV Exposure on Proliferation in the Loops
2.2.4. Effect of DON and NIV Exposure on Apoptosis in the Loops

3. Discussion
3.1. The Jejunum Explants and Loops Alternative Models Reduce the Number of Animals
3.2. Acute Exposure to NIV More Toxic in Vitro on Jejunum Mucosa than DON
3.3. Acute Exposure to NIV More Toxic in Vivo on Jejunum Mucosa than DON
3.4. DON and NIV Induced Apoptosis in Vivo on Loops
3.5. Relevance of the Results for Risk Characterization
4. Materials and Methods
4.1. Animals
4.2. Toxins
4.3. Jejunum Explants Experiment (in Vitro)
4.3.1. Jejunal Explants
4.3.2. Histological Scoring
| Score component | Criteria (severity factor) | End-point | Score |
|---|---|---|---|
| Lesional part of the Score | Enterocytes morphology (2) | Columnar epithelium | 3 |
| <50% cuboid epithelium | 2 | ||
| >50% cuboid epithelium | 1 | ||
| Flattened epithelium | 0 | ||
| Apical denudation of villi (2) | 0%–10% | 3 | |
| 11%–40% | 2 | ||
| 41%–70% | 1 | ||
| 71%–100% | 0 | ||
| Lesions of lamina propria (2) | No lesions, slight flattening of villi | 2 | |
| Localized edema and apoptosis | 1 | ||
| Multifocal edema and apoptosis | 0 | ||
| Architectural part of the Score | Villi fusion (1) | 0%–11% | 3 |
| 12%–40% | 2 | ||
| 41%–70% | 1 | ||
| 71%–100% | 0 | ||
| Number of villi (1) | ≥25 | 3 | |
| 16–24 | 2 | ||
| 5–15 | 1 | ||
| ≤4 | 0 |
4.4. Jejunum Loops Experiment (in Vivo)
4.4.1. Jejunal Loops Injection and Sampling
4.4.2. Histological Processing
4.4.3. Immunohistochemistry
4.4.4. Architectural Changes
4.4.5. Proliferative and Apoptosis Indexes
| Endpoint | Counted area (Figure 2) | Cells counts | Indexes |
|---|---|---|---|
| Proliferation | |||
| Villus tip | Total cells: lamina propria cells + enterocytes | ||
| Crypt bases | Crypt enterocytes | Proliferative index of crypt enterocytes: number of positive enterocytes/total number of enterocytes (×100) | |
| Apoptosis | |||
| Villus tip | Enterocytes Lamina propria cells (mainly immune cells) Total cells: lamina propria + enterocytes | Enterocyte apoptotic index: number of positive enterocytes/total number of enterocytes (×100) | |
| Ratio Proliferation/Apoptosis | |||
| Villus tip | Total cells: lamina propria cells + enterocytes | Total-cell proliferation to total cell apoptosis ratio | |
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smith, L.E.; Stoltzfus, R.J. Prendergast, a. Food Chain Mycotoxin Exposure, Gut Health, and Impaired Growth: A Conceptual Framework. Adv. Nutr. An Int. Rev. J. 2012, 3, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Schollenberger, M.; Müller, H.M.; Rüfle, M.; Suchy, S.; Planck, S.; Drochner, W. Survey of Fusarium toxins in foodstuffs of plant origin marketed in Germany. Int. J. Food Microbiol. 2005, 97, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Kobayashi, H.; Nagata, T.; Manabe, M. Natural occurrence of trichothecenes on lodged and water-damaged domestic rice in Japan. Shokuhin Eiseigaku Zasshi 2004, 45, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states” Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Health. B. Crit. Rev. 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, J.-C.; Tard, A.; Volatier, J.-L.; Verger, P. Estimated dietary exposure to principal food mycotoxins from the first French Total Diet Study. Food Addit. Contam. 2005, 22, 652–672. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Some Naturally Occurring Substances, Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. Monograph on the Evaluation of Carcinogenic Risks to Humans; World Health Organization, International Agency for Research on Cancer: Lyon, France, 1993; pp. 397–444. [Google Scholar]
- EFSA. Scientific Opinion on risks for animal and public health related to the presence of nivalenol in food and feed. EFSA J. 2013, 11, 1–119. [Google Scholar] [CrossRef]
- Rocha, O.; Ansari, K.; Doohan, F.M. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 2005, 22, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Van De Walle, J.; Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.-J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Yahi, N.; Younès-Sakr, L.; Boyron, M.; Caporiccio, B.; Fantini, J. Both direct and indirect effects account for the pro-inflammatory activity of enteropathogenic mycotoxins on the human intestinal epithelium: Stimulation of interleukin-8 secretion, potentiation of interleukin-1β effect and increase in the transepithelial passage of commensal bacteria. Toxicol. Appl. Pharmacol. 2008, 228, 84–92. [Google Scholar] [PubMed]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins (Basel) 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.-P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.-D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Hedman, R.; Thuvander, A.; Gadhasson, I.; Reverter, M.; Pettersson, H. Influence of dietary nivalenol exposure on gross pathology and selected immunological parameters in young pigs. Nat. Toxins 1997, 5, 238–246. [Google Scholar] [CrossRef]
- Madej, M.; Lundh, T.; Lindberg, J.E. Effect of exposure to dietary nivalenol on activity of enzymes involved in glutamine catabolism in the epithelium along the gastrointestinal tract of growing pigs. Arch. Tierernahr. 1999, 52, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Russel, W.M.S.; Burch, R.L. The Principles of Human Experimental Technique; Methuen: London, UK, 1959; pp. 54–66. [Google Scholar]
- Girard, F.; Dziva, F.; van Diemen, P.; Phillips, A.D.; Stevens, M.P.; Frankel, G. Adherence of enterohemorrhagic Escherichia coli O157, O26, and O111 strains to bovine intestinal explants ex vivo. Appl. Environ. Microbiol. 2007, 73, 3084–3090. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Berri, M.; Auray, G.; Melo, S.; Levast, B.; Virlogeux-Payant, I.; Chevaleyre, C.; Gerdts, V.; Salmon, H. Early immune response following Salmonella enterica subspecies enterica serovar Typhimurium infection in porcine jejunal gut loops. Vet. Res. 2009, 40, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Nietfeld, J.C.; Tyler, D.E.; Harrison, L.R.; Cole, J.R.; Latimer, K.S.; Crowell, W.A. Culture and morphologic features of small intestinal mucosal explants from weaned pigs. Am. J. Vet. Res. 1991, 52, 1142–1146. [Google Scholar] [PubMed]
- Basso, K.; Gomes, F.; Bracarense, A.P.L. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in E-cadherin expression. Toxins (Basel) 2013, 5, 2341–2352. [Google Scholar] [CrossRef] [PubMed]
- Lucioli, J.; Pinton, P.; Callu, P.; Laffitte, J.; Grosjean, F.; Kolf-Clauw, M.; Oswald, I.P.; Bracarense, A.P.F.R.L. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 2013, 66, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Castellote, J.; Joly, B.; Bourges-Abella, N.; Raymond-Letron, I.; Pinton, P.; Oswald, I.P. Development of a pig jejunal explant culture for studying the gastrointestinal toxicity of the mycotoxin deoxynivalenol: Histopathological analysis. Toxicol. In Vitro 2009, 23, 1580–1584. [Google Scholar] [CrossRef] [PubMed]
- Kolf-Clauw, M.; Sassahara, M.; Lucioli, J.; Rubira-Gerez, J.; Alassane-Kpembi, I.; Lyazhri, F.; Borin, C.; Oswald, I.P. The emerging mycotoxin, enniatin B1, down-modulates the gastrointestinal toxicity of T-2 toxin in vitro on intestinal epithelial cells and ex vivo on intestinal explants. Arch. Toxicol. 2013, 87, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P.F.L. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2015, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Fontanella, B.; Severino, L.; Quaroni, A.; Autore, G.; Marzocco, S. Nivalenol and Deoxynivalenol Affect Rat Intestinal Epithelial Cells: A Concentration Related Study. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Marzocco, S.; Russo, R.; Bianco, G.; Autore, G.; Severino, L. Pro-apoptotic effects of nivalenol and deoxynivalenol trichothecenes in J774A.1 murine macrophages. Toxicol. Lett. 2009, 189, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Kolf-Clauw, M.; Gauthier, T.; Abrami, R.; Abiola, F.A.; Oswald, I.P.; Puel, O. New insights into mycotoxin mixtures: The toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol. Appl. Pharmacol. 2013, 272, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.M.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Luongo, D.; de Luna, R.; Russo, R.; Severino, L. Effects of four Fusarium toxins (fumonisin B1, alpha-zearalenol, nivalenol and deoxynivalenol) on porcine whole-blood cellular proliferation. Toxicon 2008, 52, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minervini, F.; Fornelli, F.; Flynn, K.M. Toxicity and apoptosis induced by the mycotoxins nivalenol, deoxynivalenol and fumonisin B1 in a human erythroleukemia cell line. Toxicol. Vitr. 2004, 18, 21–28. [Google Scholar] [CrossRef]
- Haschek, W.M.; Rousseaux, C.G.; Wallig, M.A. Fundamentals of Toxicologic Pathology, 2nd ed.; Academic Press-Elsevier: London, UK, 2010; pp. 168–169. [Google Scholar]
- Pinton, P.; Oswald, I.P. Effect of deoxynivalenol and other type B trichothecenes on the intestine: A review. Toxins (Basel) 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, H.; Kobayashi, T.; Ryu, J.C.; Ueno, Y.; Nakamura, K.; Izumiyama, N.; Ohtsubo, K. Subchronic feeding studies with nivalenol in C57BL/6 mice. Food Chem. Toxicol. 1989, 27, 585–590. [Google Scholar] [CrossRef]
- Ryu, J.C.; Ohtsubo, K.; Izumiyama, N.; Nakamura, K.; Tanaka, T.; Yamamura, H.; Ueno, Y. The acute and chronic toxicities of nivalenol in mice. Toxicol. Sci. 1988, 11, 38–47. [Google Scholar] [CrossRef]
- Kararli, T.T. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 1995, 16, 351–380. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Flannery, B.M.; Sugita-Konishi, Y.; Watanabe, M.; Zhang, H.; Pestka, J.J. Comparison of murine anorectic responses to the 8-ketotrichothecenes 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, fusarenon X and nivalenol. Food Chem. Toxicol. 2012, 50, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Islam, Z.; Pestka, J.J. Rapid, sequential activation of mitogen-activated protein kinases and transcription factors precedes proinflammatory cytokine mRNA expression in spleens of mice exposed to the trichothecene vomitoxin. Toxicol. Sci. 2003, 72, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.M.; Seeboth, J.; Meurens, F.; Cognie, J.; Abrami, R.; Oswald, I.P.; Guzylack-Piriou, L. Deoxynivalenol as a New Factor in the Persistence of Intestinal Inflammatory Diseases: An Emerging Hypothesis through Possible Modulation of Th17-Mediated Response. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Sirot, V.; Fremy, J.M.; Leblanc, J.C. Dietary exposure to mycotoxins and health risk assessment in the second French total diet study. Food Chem. Toxicol. 2013, 52, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.C.; Wu, Z.Y.; Li, Y.S.; Zhang, F.; Sun, Z.T. Nivalenol, a main Fusarium toxin in dietary foods from high-risk areas of cancer of esophagus and gastric cardia in China, induced benign and malignant tumors in mice. Oncol. Rep. 2004, 12, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Girard-Misguich, F.; Cognie, J.; Delgado-Ortega, M.; Berthon, P.; Rossignol, C.; Larcher, T.; Melo, S.; Bruel, T.; Guibon, R.; Chérel, Y.; et al. Towards the establishment of a porcine model to study human amebiasis. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Abot, A.; Fontaine, C.; Raymond-Letron, I.; Flouriot, G.; Adlanmerini, M.; Buscato, M.; Otto, C.; Bergès, H.; Laurell, H.; Gourdy, P.; et al. The AF-1 activation function of estrogen receptor α is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology 2013, 154, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Laprie, C.; Abadie, J.; Amardeilh, M.F.; Raymond, I.; Delverdier, M. Detection of the Ki-67 proliferation associated nuclear epitope in normal canine tissues using the monoclonal antibody MIB-1. Anat. Histol. Embryol. 1998, 27, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.W.; Overend, P.; Borja, M.C.; Berdoy, M. The Design of Animal Experiments: Reducing the Use of Animals in Research through Better Experimental Design; Royal Society of Medicine Press Limited: London, UK, 2002; pp. 38–59. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheat, S.; Gerez, J.R.; Cognié, J.; Alassane-Kpembi, I.; Bracarense, A.P.F.L.; Raymond-Letron, I.; Oswald, I.P.; Kolf-Clauw, M. Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops. Toxins 2015, 7, 1945-1961. https://doi.org/10.3390/toxins7061945
Cheat S, Gerez JR, Cognié J, Alassane-Kpembi I, Bracarense APFL, Raymond-Letron I, Oswald IP, Kolf-Clauw M. Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops. Toxins. 2015; 7(6):1945-1961. https://doi.org/10.3390/toxins7061945
Chicago/Turabian StyleCheat, Sophal, Juliana R. Gerez, Juliette Cognié, Imourana Alassane-Kpembi, Ana Paula F. L. Bracarense, Isabelle Raymond-Letron, Isabelle P. Oswald, and Martine Kolf-Clauw. 2015. "Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops" Toxins 7, no. 6: 1945-1961. https://doi.org/10.3390/toxins7061945
APA StyleCheat, S., Gerez, J. R., Cognié, J., Alassane-Kpembi, I., Bracarense, A. P. F. L., Raymond-Letron, I., Oswald, I. P., & Kolf-Clauw, M. (2015). Nivalenol Has a Greater Impact than Deoxynivalenol on Pig Jejunum Mucosa in Vitro on Explants and in Vivo on Intestinal Loops. Toxins, 7(6), 1945-1961. https://doi.org/10.3390/toxins7061945