Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs
Abstract
:1. Introduction
| DON | Trial period | Biomarker | DON effects | Reference |
|---|---|---|---|---|
| 4 mg/kg feed | 37 days | Oxidative stress markers in blood (catalase (CAT), total antioxidant capacity (T-AOC), hydrogen peroxide (H2O2), nitric oxide (NO), maleic dialdehyde (MDA) and diamine oxidase (DAO)), kidney, liver and small intestine (H2O2, MDA and DAO) Intestinal morphology | DON induced oxidative stress DON increased intestinal permeability DON inhibited protein synthesis and cell proliferation | [18] |
| 2.9 mg/kg feed | 1 week | DON transport study | Dietary DON affected the jejunal transport of DON | [19] |
| 3.1 mg/kg feed | 37 days | Crypt depth Intestinal cell proliferation Immunofluorescence staining zona occludens protein-1 (ZO-1) and β-catenin | No effect on crypt depth No effect on epithelial cell proliferation No effect on apical junction proteins | [20] |
| 2.3 mg/kg feed | 35 days | Intestinal morphology/histological score jejunum Mitogen activated protein kinases (MAPK) expression in jejunum | DON induced histological lesions DON activated MAPK extracellular-signal-regulated kinases 1/2 (ERK1/2) and p38 | [21] |
| 2.2–2.9 mg/kg feed | 11 weeks | Composition and perforation of the basement membrane of intestinal villi Presence of CD16+ cells or their dendrites in the epithelium | DON increased the pore number in jejunum DON increased the number of CD16+ cells in the epithelium of the jejunum | [22] |
| 4 mg/kg feed | 30 days | Intestinal morphology Intestinal function | DON enhanced intestinal permeability, damaged villi, caused epithelial cell apoptosis and inhibited protein synthesis | [23] |
| 3 mg/kg feed | 5 weeks | Intestinal morphology, intestinal cytokine expression Tight and adherens junction protein expression (occludin (OCLN), E-cadherin) | DON induced atrophy and fusion of villi DON decreased villi height and cell proliferation in the jejunum DON reduced number of goblet cells and lymphocytes DON induced up regulation of cytokine expression in jejunum and ileum DON reduced the expression of E-cadherin and OCLN in ileum | [16] |
| 3 mg/kg feed | 10 weeks | Growth performance Histomorphometric and immuno-fluorescence investigations of small intestinal epithelium | DON decreased the feed intake (grower) DON increased the crypt depth in jejunum No effect on villus height and ZO-1 expression in jejunum and ileum | [24] |
| 2.29 mg/kg feed | 4 weeks | Intestinal morphology/histological scores | DON induced atrophy and villus fusion, necrotic debris and areas of enterocytes lyses DON caused 15% lower histological scores in jejunum | [25] |
| 2.85 mg/kg feed | 5 weeks | Claudin-4 (CLDN4) expression (Western blot, immunofluorescence staining) in jejunum | DON reduced CLDN4 expression in jejunum | [26] |
| 2.8 mg/kg feed | 4 weeks | Growth performance, intestinal microflora | DON reduced the daily weight gain (first week) Moderate effect on cultivable bacteria in the intestine | [27] |
| 1.2–2 mg/kg feed | 84 days | Gene expression in ileum | DON induced a downregulation of interleukin-1 beta (IL-1β) and IL-8 expression in ileum | [28] |
| 12 µg/kg BW/day | 42 days | Absorption, accumulation and final presence of DON in the gastrointestinal tract | Presence of DON in intestinal tissues: DON concentrations in small intestine ranged from 7.2 (in the duodenum) to 18.6 ng/g (in the ileum) and in large intestine from 1.8 (in transverse the colon) to 23.0 ng/g (in the cecum) | [29] |
| 1.5 mg/kg feed | 28 days | Weight gain, histological changes in medium jejunum, proximal ileum and mesenteric lymph nodes | DON induced a decrease in villus height of jejunum, and a reduction in crypt depth of jejunum and ileum DON induced a decrease in number of mitotic figures, goblet cells in jejunum and ileum DON induced a decrease in number of lymphocytes in jejunum DON induced significant increase in lesional score and caspase-3 positive cells in lymph nodes | [30] |
2. Results
2.1. Average Daily Gain is Decreased by 0.9 ppm DON in the Diet
Item | Start weight (kg) | End weight (kg) | Relative weight gain (% increase) | Average daily gain (kg/day) | Feed intake (kg/day) | Feed conversion ratio | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. group | Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | Mean | S.E.M. | Mean | Mean |
| Control | 8.67 | 0.48 | 10.98 | 0.53 | 27.21 | 1.82 | 0.29 | 0.01 | 0.31 | 1.12 |
| DON | 7.87 | 0.47 | 9.48 | 0.61 | 20.17 * | 1.15 | 0.20 *** | 0.01 | 0.30 | 1.57 |
2.2. Detectable DON in Plasma Levels after Bolus Administration
2.3. Low DON Levels Can Induce Histomorphological Changes in the Piglet Intestine

2.4. Several Markers for Barrier Integrity, Inflammation and Oxidative Stress in the Intestines Are Affected by the DON Diet
2.4.1. Tight Junction Proteins

2.4.2. Inflammatory Markers

2.4.3. Oxidative Stress Markers
2.4.4. Efflux Transporter

2.4.5. Proliferation and Apoptosis

3. Discussion

3.1. Growth and Performance
| Target gene | Primer sequence (5′-3′) | AT | Reference sequence | |
|---|---|---|---|---|
| Forward | Reverse | |||
| ABCB1 | TGGCAGTGGGACAGGTTAGTTC | CACGGTGCTTGAGCTGTCAATC | 65 | AY825267 |
| Caspase-3 | AGAGGGGACTGCTGTAGAACT | CCGTCTCAATCCCACAGTCC | 58.7 | NM_214131.1 |
| CLDN1 | TGGCTCCGCGTCTCAGTCC | TGCGAGGGGTGCAGGTCTAA | 65 | NM_001244539.1 |
| CLDN2 | CTCGTTGGCCTGTATCATCACC | CAGGGGGGAGTAGAAGTCCC | 63.1 | NM_001161638.1 |
| CLDN3 | AACACCATCATCCGGGACTTC | CGCGGAGTAGAGGATCTTGG | 61.2 | NM_001160075.1 |
| CLDN4 | AGGAGAGACGCTTCAATCGG | GTCCAGACACCTGAACACCG | 63.1 | NM_001161637.1 |
| CLDN5 | CTCTGCTGGTTCGCCAACA | CAGCTCGTACTTCTGCGACATG | 58.7 | NM_001161636.1 |
| COX-1 | CAAGATGGGTCCTGGCTTCA | CCATAAATGTGGCCGAGGTCTA | 64.3 | XM_001926129.4 |
| COX-2 | CATTGATGCCATGGAGCTGTA | CTCCCCAAAGATGGCATCTG | 64.3 | NM_214321.1 |
| HIF-1α | GCTTGCTCATCAGTTGCC | GCCTTCATTTCATCTTCAATATCC | 64.3 | AY485675.1 |
| HMOX1 | AGACCGCCTTCCTGCTCA | GGGTCTCTGGTCCTTAGTGTC | 64 | NM_001004027 |
| HMOX2 | GCAGCAGTTCAAGCAGTTCT | CCTCCTCCACGATCTTCTCT | 63.1 | NM_001244412.1 |
| HPRT | CTGAACGGCTTGCTCGAGAT | TCCAGCAGGTCAGCAAAGAA | 63.1 | NM_001032376.2 |
| IL-10 | CGGCGCTGTCATCAATTTCTG | CCCCTCTTGGAGCTTGCTA | 58.7 | NM_214041 |
| IL-1β | GTGCAAACTCCAGGACAAAGACCA | CACAAGCTCATGCAGAACACCAC | 61.2 | NM_214055 |
| Ki67 | TCTTGTCCCTGAATCCGCAA | TGTTTCTCTGGTTGCTTGGTTG | 61.2 | NM_001101827.1 |
| OCLN | ATCAACAAAGGCAACTCT | GCAGCAGCCATGTACTCT | 55.8 | NM_001163647.2 |
| TLR4 | CAAGGACCAGAAGCAGCTCC | GACGGCCTCGCTTATCTGAC | 63.1 | AB188301.2 |
| ZO-1 | GAGTTTGATAGTGGCGTT | GTGGGAGGATGCTGTTGT | 58.7 | XM_005659811.1 |
| ZO-2 | GCAGAGACAACCCCCACTTT | CGTTAACCATGACCACCCGA | 55.8 | NM_001206404.1 |
3.2. DON in Plasma
3.3. Effects of DON on Intestinal Morphology

3.4. Barrier Function
3.5. Immune Parameters
3.6. Cellular Oxidative Stress
3.7. Efflux Transporters
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. DON Levels in Plasma
4.4. Histomorphometric Analysis of the Small Intestine
4.5. Determination of mRNA Expression in Intestinal Samples by qRT-PCR
4.6. Western Blot Analysis
4.7. Immunohistochemistry
4.8. Statistical Analyses
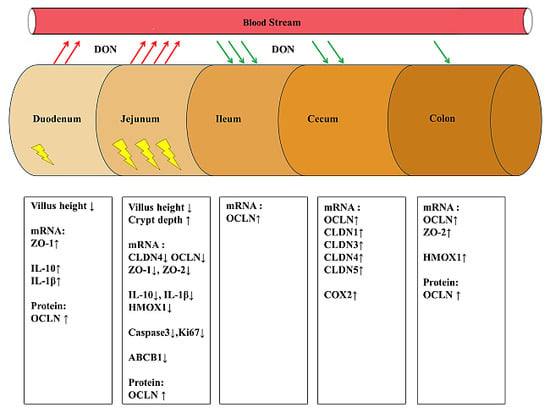
 ) are mainly observed in the jejunum (downregulation of different genes). Up arrows indicate increase and down arrows indicate decrease.
) are mainly observed in the jejunum (downregulation of different genes). Up arrows indicate increase and down arrows indicate decrease.
 ) are mainly observed in the jejunum (downregulation of different genes). Up arrows indicate increase and down arrows indicate decrease.
) are mainly observed in the jejunum (downregulation of different genes). Up arrows indicate increase and down arrows indicate decrease.
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission related to Deoxynivalenol (DON) as undesirable substance in animal feed. EFSA J. 2004, 73, 1–42. [Google Scholar]
- Grenier, B.; Applegate, T.J. Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins (Basel) 2013, 5, 396–430. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Oswald, I. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins (Basel) 2014, 6, 1615–1643. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and potential effects on humans. J. Toxicol. Environ. Heal. 2005, 8, 39–69. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M. Mycotoxins in animals: Occurrence, effects, prevention and management. J. Toxicol. Environ. Heal. Sci. 2012, 4, 13–28. [Google Scholar] [CrossRef]
- Akbari, P.; Braber, S.; Gremmels, H.; Koelink, P.J.; Verheijden, K.A.T.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol: A trigger for intestinal integrity breakdown. FASEB J. 2014, 28, 2414–2429. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M. From the gut to the brain: Journey and pathophysiological effects of the food-associated trichothecene mycotoxin deoxynivalenol. Toxins (Basel) 2013, 5, 784–820. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins (Basel) 2012, 4, 1120–1138. [Google Scholar] [CrossRef] [PubMed]
- Amuzie, C.J.; Pestka, J.J. Suppression of insulin-like growth factor acid-labile subunit expression—A novel mechanism for deoxynivalenol-induced growth retardation. Toxicol. Sci. 2010, 113, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Pestka, J.J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol-induced proinflammatory gene expression: Mechanisms and pathological sequelae. Toxins (Basel) 2010, 2, 1300–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Harkema, J.R.; Hotchkiss, J.A.; Yan, D.; Roth, R.A.; Pestka, J.J. Lipopolysaccharide and the trichothecene vomitoxin (deoxynivalenol) synergistically induce apoptosis in murine lymphoid organs. Toxicol. Sci. 2000, 53, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Sundstøl Eriksen, G.; Pettersson, H.; Lundh, T. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food Chem. Toxicol. 2004, 42, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Bracarense, A.P.F.L.; Lucioli, J.; Grenier, B.; Drociunas Pacheco, G.; Moll, W.D.; Schatzmayr, G.; Oswald, I.P. Chronic ingestion of deoxynivalenol and fumonisin, alone or in interaction, induces morphological and immunological changes in the intestine of piglets. Br. J. Nutr. 2012, 107, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xiao, H.; Ren, W.; Yin, J.; Tan, B.; Liu, G.; Li, L.; Nyachoti, C.M.; Xiong, X.; Wu, G. Therapeutic effects of glutamic Acid in piglets challenged with deoxynivalenol. PLoS ONE 2014, 9, e100591. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A.; Dänicke, S.; Kersten, S.; Breves, G. Intestinal transport of deoxynivalenol across porcine small intestines. Arch. Anim. Nutr. 2013, 67, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Klunker, L.; Kahlert, S. Deoxynivalenol and E. coli lipopolysaccharide alter epithelial proliferation and spatial distribution of apical junction proteins along the small intestinal axis. J. Anim. Sci. 2013, 91, 276–285. [Google Scholar]
- Lucioli, J.; Pinton, P.; Callu, P.; Laffitte, J.; Grosjean, F.; Kolf-Clauw, M.; Oswald, I.P.; Bracarense, A.P. The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: Interest of ex vivo models as an alternative to in vivo experiments. Toxicon 2013, 66, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Nossol, C.; Diesing, A.K.; Kahlert, S.; Kersten, S.; Kluess, J.; Ponsuksili, S.; Hartig, R.; Wimmers, K.; Dänicke, S.; Rothkötter, H.J. Deoxynivalenol affects the composition of the basement membrane proteins and influences en route the migration of CD16+ cells into the intestinal epithelium. Mycotoxin Res. 2013, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Tan, B.E.; Wu, M.M.; Yin, Y.L.; Li, T.J.; Yuan, D.X.; Li, L. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: II. Intestinal morphology and function. J. Anim. Sci. 2013, 91, 4750–4756. [Google Scholar]
- Dänicke, S.; Brosig, B.; Raja, L.; Kahlert, S.; Kluess, J.; Döll, S.; Valenta, H.; Rothkötter, H. Systemic and local effects of the Fusarium toxin deoxynivalenol (DON) are not alleviated by dietary supplementation of humic substances (HS). Food Chem. Toxicol. 2012, 50, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Tsybulskyy, D.; Lucioli, J.; Laffitte, J.; Callu, P.; Lyazhri, F.; Grosjean, F.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. Toxicity of deoxynivalenol and its acetylated derivatives on the intestine: Differential effects on morphology, barrier function, tight junction proteins, and mitogen-activated protein kinases. Toxicol. Sci. 2012, 130, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Pinton, P.; Nougayrède, J.P.; del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Bracarense, A.P.; Kolf-Clauw, M.; Oswald, I.P. The food contaminant deoxynivalenol, decreases intestinal barrier permeability and reduces claudin expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waché, Y.J.; Valat, C.; Postollec, G.; Bougeard, S.; Burel, C.; Oswald, I.P.; Fravalo, P. Impact of deoxynivalenol on the intestinal microflora of pigs. Int. J. Mol. Sci. 2009, 10, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Reiter, M.; Pfaffl, M.W.; Meyer, H.H.D.; Bauer, J.; Meyer, K.H.D. Expression of immune relevant genes in pigs under the influence of low doses of deoxynivalenol (DON). Mycotoxin Res. 2011, 27, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Beszterda, M.; Kostecki, M.; Zielonka, Ł.; Goliński, P.; Gajęcki, M. Deoxynivalenol in the gastrointestinal tract of immature gilts under per os toxin application. Toxins (Basel) 2014, 6, 973–987. [Google Scholar]
- Gerez, J.R.; Pinton, P.; Callu, P.; Grosjean, F.; Oswald, I.P.; Bracarense, A.P. Deoxynivalenol alone or in combination with nivalenol and zearalenone induce systemic histological changes in pigs. Exp. Toxicol. Pathol. 2014, 67, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Kersten, S. Humic substances failed to prevent the systemic absorption of deoxynivalenol (DON) and its adverse effects on piglets. Mycotoxin Res. 2012, 28, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Goyarts, T.; Valenta, H. On the specific and unspecific effects of a polymeric glucomannan mycotoxin adsorbent on piglets when fed with uncontaminated or with Fusarium toxins contaminated diets. Arch. Anim. Nutr. 2007, 61, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wu, M.; Tan, B. Effects of composite antimicrobial peptides in weanling piglets challenged with deoxynivalenol: I. Growth performance, immune function, and antioxidation capacity. J. Anim. Sci. 2013, 91, 4772–4780. [Google Scholar]
- Accensi, F.; Pinton, P.; Callu, P.; Abella Bourges, N.; Guelfi, J.F.; Grosjean, F.; Oswald, I.P. Ingestion of low doses of deoxynivalenol does not affect hematological, biochemical, or immune responses of piglets. J. Anim. Sci. 2006, 84, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Dänicke, S.; Ueberschär, K.H.; Valenta, H.; Schnurrbusch, U.; Ganter, M.; Klobasa, F.; Flachowsky, G. Effects of graded levels of Fusarium toxin contaminated maize in diets for female weaned piglets. Arch. Anim. Nutr. 2003, 57, 311–334. [Google Scholar] [CrossRef]
- Friend, D.W.; Trenholm, H.; Elliot, J. Effect of feeding vomitoxin-contaminated wheat to pigs. Can. J. Anim. Sci. 1982, 62, 1211–1222. [Google Scholar] [CrossRef]
- Pestka, J.J.; Amuzie, C.J. Tissue Distribution and Proinflammatory Cytokine Gene Expression Following Acute Oral Exposure to Deoxynivalenol: Comparison of Weanling and Adult Mice. Food Chem. Toxicol. 2008, 46, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Beyer, M.; Breves, G.; Valenta, H.; Humpf, H.-U. Effects of oral exposure of pigs to deoxynivalenol (DON) sulfonate (DONS) as the non-toxic derivative of DON on tissue residues of DON and de-epoxy-DON and on DONS blood levels. Food Addit. Contam. 2010, 27, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Ganter, M.; Brosig, B.; Kersten, S.; Diesing, A.K.; Kahlert, S.; Panther, P.; Kluess, J.; Rothkötter, H.J. Lipopolysaccharides (LPS) modulate the metabolism of deoxynivalenol (DON) in the pig. Mycotoxin Res. 2014, 30, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Diesing, A.K.; Nossol, C.; Dänicke, S.; Walk, N.; Post, A.; Kahlert, S.; Rothkötter, H.J.; Kluess, J. Vulnerability of polarised intestinal porcine epithelial cells to mycotoxin deoxynivalenol depends on the route of application. PLoS ONE 2011, 6, e17472. [Google Scholar] [CrossRef] [PubMed]
- Blikslager, A.T.; Moeser, A.J.; Gookin, J.L.; Jones, S.L.; Odle, J. Restoration of barrier function in injured intestinal mucosa. Physiol. Rev. 2007, 87, 545–564. [Google Scholar] [CrossRef] [PubMed]
- Awad, W.A.; Ghareeb, K.; Zentek, J. Mechanisms underlying the inhibitory effect of the feed contaminant deoxynivalenol on glucose absorption in broiler chickens. VET J. 2014, 202, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Mahfoud, R.; Garmy, N.; Fantini, J. The mycotoxin deoxynivalenol affects nutrient absorption in human intestinal epithelial cells. J. Nutr. 2002, 132, 2723–2731. [Google Scholar] [PubMed]
- Awad, W.A.; Ghareeb, K.; Abdel-Raheem, S.; Böhm, J. Effects of dietary inclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009, 88, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R.; Thompson, M.J.; Atwood, C.S.; Bird, P.H.; Williams, I.H.; Hartmann, P.E. Maintenance of villus height and crypt depth, and enhancement of disaccharide digestion and monosaccharide absorption, in piglets fed on cows’ whole milk after weaning. Br. J. Nutr. 1996, 76, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Cummins, P.M. Occludin: One protein, many forms. Mol. Cell. Biol. 2012, 32, 242–250. [Google Scholar] [CrossRef] [PubMed]
- De Walle, J.; Van Sergent, T.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol affects in vitro intestinal epithelial cell barrier integrity through inhibition of protein synthesis. Toxicol. Appl. Pharmacol. 2010, 245, 291–298. [Google Scholar]
- Jeong Gu, M.; Kwang, S.; Park, S.M.; Lee, I.K.; Yun, C.-H. Bacillus subtilis Protects Porcine Intestinal Barrier from Deoxynivalenol via Improved Zonula Occludens-1 Expression. Asian Australas. J. Anim. Sci. 2014, 27, 580–586. [Google Scholar]
- Lessard, M.; Savard, C.; Deschene, K.; Lauzon, K.; Pinilla, V.A.; Gagnon, C.A.; Lapointe, J.; Guay, F.; Chorfi, Y. Impact of deoxynivalenol (DON) contaminated feed on intestinal integrity and immune response in swine. Food Chem. Toxicol. 2015, 80, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.L.; Van Itallie, C.M.; Rasmussen, J.E.; Anderson, J.M. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr. Patterns 2006, 6, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Dänicke, S.; Valenta, H.; Döll, S. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. Arch. Anim. Nutr. 2004, 58, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Diesing, A.K.; Nossol, C.; Ponsuksili, S.; Wimmers, K.; Kluess, J.; Walk, N.; Post, A.; Rothkötter, H.J.; Kahlert, S. Gene regulation of intestinal porcine epithelial cells IPEC-J2 is dependent on the site of deoxynivalenol toxicological action. PLoS ONE 2012, 7, e34136. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J.; Zhou, H.R.; Moon, Y.; Chung, Y.J. Cellular and molecular mechanisms for immune modulation by deoxynivalenol and other trichothecenes: Unraveling a paradox. Toxicol. Lett. 2004, 153, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yan, D.; Pestka, J. Induction of cytokine gene expression in mice after repeated and subchronic oral exposure to vomitoxin (Deoxynivalenol): Differential toxin-induced hyporesponsiveness and recovery. Toxicol. Appl. Pharmacol. 1998, 151, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Amuzie, C.J.; Harkema, J.R.; Pestka, J.J. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: Comparison of nasal vs. oral exposure. Toxicology 2008, 248, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Mikami, O.; Yamaguchi, H.; Murata, H.; Nakajima, Y.; Miyazaki, S. Induction of apoptotic lesions in liver and lymphoid tissues and modulation of cytokine mRNA expression by acute exposure to deoxynivalenol in piglets. J. Vet. Sci. 2010, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Yan, D.; Pestka, J.J. Differential cytokine mRNA expression in mice after oral exposure to the trichothecene vomitoxin (deoxynivalenol): Dose response and time course. Toxicol. Appl. Pharmacol. 1997, 144, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, R.; Devaraj, S.N.; Padma, V.V. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: Prevention of NF-kappaB nuclear localization and down regulation of NF-kappaB and Cyclo-Oxygenase-2 expression. Free Radic. Biol. Med. 2010, 49, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, J.; During, A.; Piront, N.; Toussaint, O.; Schneider, Y.J.; Larondelle, Y. Physio-pathological parameters affect the activation of inflammatory pathways by deoxynivalenol in Caco-2 cells. Toxicol. Vitr. 2010, 24, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Döll, S.; Schrickx, J.A.; Dänicke, S.; Fink-Gremmels, J. Deoxynivalenol-induced cytotoxicity, cytokines and related genes in unstimulated or lipopolysaccharide stimulated primary porcine macrophages. Toxicol. Lett. 2009, 184, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Pestka, J.J. Vomitoxin-induced cyclooxygenase-2 gene expression in macrophages mediated by activation of ERK and p38 but not JNK mitogen-activated protein kinases. Toxicol. Sci. 2002, 69, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.H.; Wang, X.; Yang, W.; Nüssler, A.K.; Xiong, L.Y.; Kuča, K.; Dohnal, V.; Zhang, X.J.; Yuan, Z.H. Oxidative stress-mediated cytotoxicity and metabolism of T-2 toxin and deoxynivalenol in animals and humans: An update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jiang, L.; Geng, C.; Cao, J.; Zhong, L. The role of oxidative stress in deoxynivalenol-induced DNA damage in HepG2 cells. Toxicon 2009, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Dinu, D.; Bodea, G.O.; Ceapa, C.D.; Munteanu, M.C.; Roming, F.I.; Serban, A.I.; Hermenean, A.; Costache, M.; Zarnescu, O.; Dinischiotu, A. Adapted response of the antioxidant defense system to oxidative stress induced by deoxynivalenol in Hek-293 cells. Toxicon 2011, 57, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Sheth, P.; Basuroy, S.; Li, C.; Naren, A.P.; Rao, R.K. Role of phosphatidylinositol 3-kinase in oxidative stress-induced disruption of tight junctions. J. Biol. Chem. 2003, 278, 49239–49245. [Google Scholar] [CrossRef] [PubMed]
- Basuroy, S.; Seth, A.; Elias, B.; Naren, A.P.; Rao, R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem. J. 2006, 393, 69–77. [Google Scholar] [PubMed]
- Eriksen, G.S.; Pettersson, H.; Lindberg, J.E. Absorption, metabolism and excretion of 3-acetyl don in pigs. Arch. Anim. Nutr. 2003, 57, 335–345. [Google Scholar] [CrossRef]
- Videmann, B.; Tep, J.; Cavret, S.; Lecoeur, S. Epithelial transport of deoxynivalenol: Involvement of human P-glycoprotein (ABCB1) and multidrug resistance-associated protein 2 (ABCC2). Food Chem. Toxicol. 2007, 45, 1938–1947. [Google Scholar] [CrossRef] [PubMed]
- Van der Heyden, S.; Goossens, J.; Vandenbroucke, V.; Vercauteren, G.; Chiers, K.; Pasmans, F.; Haesebrouck, F.; de Backer, P.; Croubels, S.; Ducatelle, R. Reduced expression of intestinal p-glycoprotein following ingestion of deoxynivalenol (DON) contaminatad feed in pigs. J. Comp. Pathol. 2009, 141, 272. [Google Scholar] [CrossRef]
- Pié, S.; Lallès, J.P.; Blazy, F.; Laffitte, J.; Sève, B.; Oswald, I.P. Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J. Nutr. 2004, 134, 641–647. [Google Scholar] [PubMed]
- Janes, W.; Schuster, M. Determination of Deoxynivalenol (DON) in Blood, Bile, Urine and Excrement Samples from Swine Using Immunoaffinity Chromatography and LC-UV-Detection. Mycotoxin Res. 2001, 17, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Nygard, A.B.; Jørgensen, C.B.; Cirera, S.; Fredholm, M. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol. Biol. 2007, 8, 67. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins 2015, 7, 2071-2095. https://doi.org/10.3390/toxins7062071
Alizadeh A, Braber S, Akbari P, Garssen J, Fink-Gremmels J. Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins. 2015; 7(6):2071-2095. https://doi.org/10.3390/toxins7062071
Chicago/Turabian StyleAlizadeh, Arash, Saskia Braber, Peyman Akbari, Johan Garssen, and Johanna Fink-Gremmels. 2015. "Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs" Toxins 7, no. 6: 2071-2095. https://doi.org/10.3390/toxins7062071
APA StyleAlizadeh, A., Braber, S., Akbari, P., Garssen, J., & Fink-Gremmels, J. (2015). Deoxynivalenol Impairs Weight Gain and Affects Markers of Gut Health after Low-Dose, Short-Term Exposure of Growing Pigs. Toxins, 7(6), 2071-2095. https://doi.org/10.3390/toxins7062071