Higher Fusarium Toxin Accumulation in Grain of Winter Triticale Lines Inoculated with Fusarium culmorum as Compared with Wheat †
Abstract
:1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Randhawa, H.; Bona, L.; Graf, R.J. Triticale breeding—Progress and prospect. In Triticale; Eudes, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 15–32. [Google Scholar]
- Arseniuk, E.; Oleksiak, T. Production and breeding of cereals in Poland. In Proceedings of the 5th International Triticale Symposium, Radzików, Poland, 30 June–5 July 2002; pp. 11–20.
- Arseniuk, E.; Góral, T. Triticale biotic stresses—Known and novel foes. In Triticale; Eudes, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 83–108. [Google Scholar]
- Audenaert, K.; Troch, V.; Landschoot, S.; Haesaert, G. Biotic stresses in the anthropogenic hybrid triticale (× Triticosecale Wittmack): Current knowledge and breeding challenges. Eur. J. Plant Pathol. 2014, 140, 615–630. [Google Scholar] [CrossRef]
- Arseniuk, E. Triticale abiotic stresses—An overview. In Triticale; Eudes, F., Ed.; Springer: Cham, Switzerland, 2015; pp. 69–81. [Google Scholar]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Siou, D.; Gélisse, S.; Laval, V.; Repinçay, C.; Canalès, R.; Suffert, F.; Lannou, C. Effect of wheat spike infection timing on Fusarium head blight development and mycotoxin accumulation. Plant Pathol. 2014, 63, 390–399. [Google Scholar] [CrossRef]
- Mesterházy, Á. Types and components of resistance to Fusarium head blight of wheat. Plant Breed. 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Foroud, N.A.; Eudes, F. Trichothecenes in cereal grains. Int. J. Mol. Sci. 2009, 10, 147–173. [Google Scholar] [CrossRef] [PubMed]
- Kalih, R.; Maurer, H.P.; Miedaner, T. Genetic architecture of Fusarium head blight resistance in four winter triticale populations. Phytopathology 2015, 105, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Faris, J.D.; Li, W.L.; Liu, D.J.; Chen, P.D.; Gill, B.S. Candidate gene analysis of quantitative disease resistance in wheat. Theor. Appl. Genet. 1999, 98, 219–225. [Google Scholar]
- Chen, J.; Griffey, C.A.; Saghai Maroof, M.A.; Stromberg, E.L.; Biyashev, R.M.; Zhao, W.; Chappell, M.R.; Pridgen, T.H.; Dong, Y.; Zeng, Z. Validation of two major quantitative trait loci for Fusarium head blight resistance in Chinese wheat line W14. Plant Breed. 2006, 125, 99–101. [Google Scholar] [CrossRef]
- Miedaner, T.; Wilde, F.; Steiner, B.; Buerstmayr, H.; Korzun, V.; Ebmeyer, E. Stacking quantitative trait loci (QTL) for Fusarium head blight resistance from non-adapted sources in an European elite spring wheat background and assessing their effects on deoxynivalenol (DON) content and disease severity. Theor. Appl. Genet. 2006, 112, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Monger, W.; Ritieni, A.; Nicholson, P. Effect of temperature and duration of wetness during initial infection periods on disease development, fungal biomass and mycotoxin concentrations on wheat inoculated with single, or combinations of, Fusarium species. Plant Pathol. 2007, 56, 943–956. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Hazel, C.M.; Patel, S. Influence of processing on trichothecene levels. Toxicol. Lett. 2004, 153, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Cowger, C.; Arrellano, C. Plump kernels with high deoxynivalenol linked to late Gibberella zeae infection and marginal disease conditions in winter wheat. Phytopathology 2010, 100, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Miedaner, T.; Reinbrecht, C.; Lauber, U.; Schollenberger, M.; Geiger, H.H. Effects of genotype and genotype-environment interaction on deoxynivalenol accumulation and resistance to Fusarium head blight in rye, triticale, and wheat. Plant Breed. 2001, 120, 97–105. [Google Scholar] [CrossRef]
- Miedaner, T.; Heinrich, N.; Schneider, B.; Oettler, G.; Rohde, S.; Rabenstein, F. Estimation of deoxynivalenol (DON) content by symptom rating and exoantigen content for resistance selection in wheat and triticale. Euphytica 2004, 139, 123–132. [Google Scholar] [CrossRef]
- Langevin, F.; Eudes, F.; Comeau, A. Effect of trichothecenes produced by Fusarium graminearum during Fusarium head blight development in six cereal species. Eur. J. Plant Pathol. 2004, 110, 735–746. [Google Scholar] [CrossRef]
- Veitch, R.S.; Caldwell, C.D.; Martin, R.A.; Lada, R.; Salmon, D.; Anderson, D.M.; MacDonald, D. Susceptibility of winter and spring triticales to Fusarium head blight and deoxynivalenol accumulation. Can. J. Plant Sci. 2008, 88, 783–788. [Google Scholar] [CrossRef]
- Arseniuk, E.; Góral, T.; Czembor, H.J. Reaction of triticale, wheat and rye accessions to graminaceous Fusarium spp. infection at the seedling and adult plant growth stages. Euphytica 1993, 70, 175–183. [Google Scholar] [CrossRef]
- Góral, T.; Cichy, H.; Buśko, M.; Perkowski, J. Resistance to head blight and mycotoxins concentrations in kernels of Polish winter triticale lines and cultivars inoculated with Fusarium culmorum. In Proceedings of the 5th International Triticale Symposium, Radzików, Poland, 30 June–5 July 2002; pp. 501–507.
- Randhawa, H.; Eudes, F.; Beres, B.; Graf, R.; Fedak, G.; Comeau, A.; Langevin, F.; Dion, Y.; Pozniak, C. Integrated approaches for triticale breeding. In Proceedings of the 8th International Triticale Symposium, Ghent, Belgium, 10–14 June 2013; p. 29.
- Comeau, A.; Langevin, F.; Savard, M.E.; Gilber, J.; Dion, Y.; Rioux, S.; Martin, R.A.; Haber, S.; Voldeng, H.; Fedak, G.; et al. Improving Fusarium head blight resistance in bread wheat and triticale for Canadian needs. Cereal Res. Commun. 2008, 36, 91–92. [Google Scholar]
- Langevin, F.; Eudes, F.; Comeau, A.; Dion, Y.; Rioux, S.; Randhawa, H.; Fedak, G.; Cao, W.; Gilbert, J.; Lachance, C.; et al. Sources of type II Fusarium resistance for triticale breeding. In Proceedings of the 6th Canadian Workshop on Fusarium Head Blight, Ottawa, ON, Canada, 1–4 November 2009; p. 66.
- Jackowiak, H.; Packa, D.; Wiwart, M.; Perkowski, J. Scanning electron microscopy of Fusarium damaged kernels of spring wheat. Int. J. Food Microbiol. 2005, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Packa, D.; Jackowiak, H.; Góral, T.; Wiwart, M.; Perkowski, J. Scanning electron microscopy of Fusarium-infected kernels of winter triticale (× Triticosecale Wittmack). Seed Sci. Biotechnol. 2008, 2, 27–31. [Google Scholar]
- Larter, E.N.; Shebeski, L.H.; McGinnis, R.C.; Evans, L.E.; Kaltsikes, P.J. Rosner, a hexaploid triticale cultivar. Can. J. Plant Sci. 1970, 50, 122–124. [Google Scholar] [CrossRef]
- Merker, A. Chromosome composition of hexaploid triticale. Hereditas 1975, 80, 41–52. [Google Scholar] [CrossRef]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Sodkiewicz, W.; Strzembicka, A. Application of Triticum monococcum for the improvement of triticale resistance to leaf rust (Puccinia triticina). Plant Breed. 2004, 123, 39–42. [Google Scholar] [CrossRef]
- Kwiatek, M.; Wiśniewska, H.; Apolinarska, B. Cytogenetic analysis of Aegilops chromosomes, potentially usable in triticale (× Triticosecale Witt.) breeding. J. Appl. Genet. 2013, 54, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Salmanowicz, B.P.; Langner, M.; Wiśniewska, H.; Apolinarska, B.; Kwiatek, M.; Błaszczyk, L. Molecular, physicochemical and rheological characteristics of introgressive Triticale/Triticum monococcum ssp. monococcum lines with wheat 1D/1A chromosome substitution. Int. J. Mol. Sci. 2013, 14, 15595–15614. [Google Scholar] [PubMed]
- Dürr, S.R. Evaluation of Three Winter Triticale (× Triticosecale Wittmack) Populations and a Collection of Triticale Cultivars and Breeding Lines for Resistance against Fusarium Head Blight. Master’s Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, October 2014. [Google Scholar]
- Góral, T.; Buśko, M.; Cichy, H.; Jackowiak, H.; Perkowski, J. Resistance of winter triticale lines and cultivars to Fusarium head blight and deoxynivalenol accumulation in kernels. J. Appl. Genet. 2002, 43, 237–248. [Google Scholar]
- Góral, T.; Ochodzki, P. Resistance of Polish winter triticale cultivars to Fusarium head blight and accumulation of Fusarium-myctoxins in grain. In Proceedings of the 6th International Triticale Symposium, Stellenbosch, South Africa, 3–7 September 2006; Botes, W.C., Boros, D., Darvey, N., Gustafson, P., Jessop, R., Marais, G.F., Oettler, G., Salmon, D., Eds.; Stellenbosch University: Stellenbosch, South Africa, 2007; pp. 140–143. [Google Scholar]
- Góral, T.; Ochodzki, P. Effect of severity of Fusarium head blight and kernel infection with Fusarium culmorum on mycotoxin content in grain of winter wheat cultivars. In Proceedings of the 28th Mykotoxin-Workshop, Bydgoszcz, Poland, 29–31 May 2006; Grajewski, J., Twaruzek, M., Szymanska, A., Eds.; Kazimierz Wielki University: Bydgoszcz, Poland, 2006; p. 84. [Google Scholar]
- Clarke, J.M.; Depauw, R.M. Water imbibition rate of wheat kernels as affected by kernel color, weather damage, and method of threshing. Can. J. Plant Sci. 1989, 69, 1–7. [Google Scholar] [CrossRef]
- Wiwart, M.; Moś, M.; Wójtowicz, T. Studies on the imbibition of triticale kernels with a different degree of sprouting, using digital shape analysis. Plant Soil Environ. 2006, 52, 328–334. [Google Scholar]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Walentyn-Góral, D.; Kwiatek, M. Reaction of winter triticale breeding lines to Fusarium head blight and accumulation of Fusarium metabolites in grain in two environments under drought conditions. Cereal Res. Commun. 2013, 41, 106–115. [Google Scholar] [CrossRef]
- Góral, T. Evaluation of resistance of breeding lines of wheat and triticale to Fusarium head blight, snow mold, and leaf rust in Radzików in 2006. Short communication. Biul. IHAR 2007, 246, 31–44. [Google Scholar]
- Góral, T. Resistance of winter triticale cultivars to Fusarium head blight caused by Fusarium culmorum. Biul. IHAR 2009, 254, 41–50. [Google Scholar]
- Wiśniewska, H.; Góral, T.; Ochodzki, P.; Walentyn-Góral, D.; Kwiatek, M.; Majka, M.; Grzeszczak, I.; Belter, J.; Banaszak, Z.; Pojmaj, M.; et al. Resistance of winter triticale breeding lines to Fusarium head blight. Biul. IHAR 2014, 271, 29–43. [Google Scholar]
- Wiśniewska, H.; Góral, T.; Ochodzki, P.; Walentyn-Góral, D.; Kwiatek, M.; Majka, M.; Belter, J.; Banaszak, Z.; Pojmaj, M.; Kurleto, D.; et al. Resistance of winter triticale breeding lines to infection of spike with Fusarium culmorum. Biul. IHAR 2015, 276, 39–56. [Google Scholar]
- Cowger, C.; Arellano, C. Fusarium graminearum infection and deoxynivalenol concentrations during development of wheat spikes. Phytopathology 2013, 103, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Buerstmayr, H.; Krska, R.; Schuhmacher, R.; Grausgruber, H.; Ruckenbauer, P. The effect of inoculation treatment and long-term application of moisture on Fusarium head blight symptoms and deoxynivalenol contamination in wheat grains. Eur. J. Plant Pathol. 2004, 110, 299–308. [Google Scholar] [CrossRef]
- Imathiu, S.; Edwards, S.; Ray, R.; Back, M. Review article: Artificial inoculum and inoculation techniques commonly used in the investigation of Fusarium head blight in cereals. Acta Phytopathol. Entomol. Hung. 2014, 49, 129–139. [Google Scholar] [CrossRef]
- Kharbikar, L.L.; Dickin, E.T.; Edwards, S.G. Impact of post-anthesis rainfall, fungicide and harvesting time on the concentration of deoxynivalenol and zearalenone in wheat. Food Addit. Contam. A 2015, 32, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Somers, D.J.; Fedak, G.; Savard, M. Molecular mapping of novel genes controlling Fusarium head blight resistance and deoxynivalenol accumulation in spring wheat. Genome 2003, 46, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Boutigny, A.-L.; Richard-Forget, F.; Barreau, C. Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 2008, 121, 411–423. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, R. Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Crews, C.; Dall’Asta, C.; De Saeger, S.; Haesaert, G.; Karlovsky, P.; Oswald, I.P.; Seefelder, W.; Speijers, G.; Stroka, J. Masked mycotoxins: A review. Mol. Nutr. Food Res. 2013, 57, 165–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, P.H.; Nielsen, K.F.; Ghorbani, F.; Spliid, N.H.; Nielsen, G.C.; Jørgensen, L.N. Occurrence of different trichothecenes and deoxynivalenol-3-β-d-glucoside in naturally and artificially contaminated Danish cereal grains and whole maize plants. Mycotoxin Res. 2012, 28, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Oettler, G.; Heinrich, N.; Miedaner, T. Estimates of additive and dominance effects for Fusarium head blight resistance of winter triticale. Plant Breed. 2004, 123, 525–530. [Google Scholar] [CrossRef]
- Oettler, G.; Wahle, G. Genotypic and environmental variation of resistance to head blight in triticale inoculated with Fusarium culmorum. Plant Breed. 2001, 120, 297–300. [Google Scholar] [CrossRef]
- Alexander, N.J.; McCormick, S.P.; Waalwijk, C.; van der Lee, T.; Proctor, R.H. The genetic basis for 3-ADON and 15-ADON trichothecene chemotypes in Fusarium. Fungal Genet. Biol. 2011, 48, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Schmeitzl, C.; Warth, B.; Fruhmann, P.; Michlmayr, H.; Malachová, A.; Berthiller, F.; Schuhmacher, R.; Krska, R.; Adam, G. The metabolic fate of deoxynivalenol and its acetylated derivatives in a wheat suspension culture: Identification and detection of DON-15-O-glucoside, 15-acetyl-DON-3-O-glucoside and 15-acetyl-DON-3-sulfate. Toxins 2015, 7, 3112–3126. [Google Scholar] [CrossRef] [PubMed]
- Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Comparative mycotoxin profiles of Gibberella zeae populations from barley, wheat, potatoes, and sugar beets. Appl. Environ. Microbiol. 2008, 74, 6513–6520. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.A.; Bass, C.; Baldwin, T.K.; Chen, H.; Massot, F.; Carion, P.W.C.; Urban, M.; van de Meene, A.M.L.; Hammond-Kosack, K.E. Characterisation of the Fusarium graminearum-wheat floral interaction. J. Pathog. 2011, 2011, 626345. [Google Scholar] [PubMed]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Koutnik, A.; Steiner, B.; Buerstmayr, H.; Berthiller, F.; Schuhmacher, R.; Maier, F.; Schäfer, W. Investigations on the ability of Fhb1 to protect wheat against nivalenol and deoxynivalenol. Cereal Res. Commun. 2008, 36, 429–435. [Google Scholar] [CrossRef]
- Yoshida, M.; Nakajima, T. Deoxynivalenol and nivalenol accumulation in wheat infected with Fusarium graminearum during grain development. Phytopathology 2010, 100, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Siou, D.; Gélisse, S.; Laval, V.; Elbelt, S.; Repinçay, C.; Bourdat-Deschamps, M.; Suffert, F.; Lannou, C. Interactions between head blight pathogens: Consequences for disease development and toxin production in wheat spikes. Appl. Environ. Microbiol. 2015, 81, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and oxidative stress indicators in winter wheat inoculated with Fusarium graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Brinkmeyer, U.; Dänicke, S.; Valenta, H.; Flachowsky, G. Progression of deoxynivalenol and zearalenone concentrations in straw of wheat infected artificially with Fusarium culmorum. Mycotoxin Res. 2005, 21, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Argyris, J.; Van Sanford, D.; TeKrony, D. Fusarium graminearum infection during wheat seed development and its effect on seed quality. Crop Sci. 2003, 43, 1782–1788. [Google Scholar] [CrossRef]


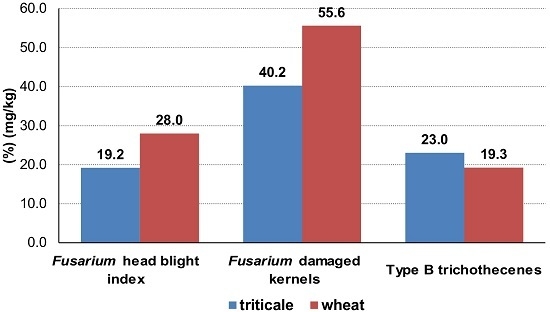
| Cereal | FHB Index (%) | FDK (%) | DON (mg/kg) | 3AcDON (mg/kg) | 15AcDON (mg/kg) | NIV (mg/kg) | TCT B (mg/kg) | ZEN (mg/kg) |
|---|---|---|---|---|---|---|---|---|
| Wheat (n = 34) | 28.0 b | 55.6 b | 11.65 a ** | 2.00 a | 1.47 a ** | 4.13 a * | 19.25 a ** | 0.60 a |
| Triticale (n = 32) | 19.2 a | 40.2 a | 14.12 b ** | 1.82 a | 1.91 b ** | 5.19 b * | 23.03 b ** | 0.66 a |
| Variables | FHB Index | FDK | DON | 3AcDON | 15AcDON | NIV | TCT B a | ZEN |
|---|---|---|---|---|---|---|---|---|
| FHB index | - | 0.604 *** | 0.538 *** | 0.630 *** | 0.333 ns | 0.326 ns | 0.576 *** | 0.412 * |
| FDK | 0.408 b,* | - | 0.451 ** | 0.529 *** | 0.336 * | 0.314 ns | 0.504 ** | 0.233 ns |
| DON | 0.398 * | 0.214 ns | - | 0.845 *** | 0.889 *** | 0.251 ns | - c | -0.014 ns |
| 3AcDON | 0.536 ** | 0.347 ns | 0.885 *** | - | 0.736 *** | 0.262 ns | - | 0.170 ns |
| 15AcDON | 0.267 ns | 0.232 ns | 0.890 *** | 0.804 *** | - | 0.057 ns | - | -0.165 ns |
| NIV | 0.180 ns | 0.465 ** | -0.406 * | -0.262 ns | -0.339 ns | - | - | 0.406 * |
| TCT B a | 0.357 * | 0.451 ** | - | - | - | - | - | 0.115 ns |
| ZEN | 0.166 ns | 0.490 ** | 0.350 * | 0.472 ** | 0.285 ns | 0.295 ns | 0.501 ** | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Góral, T.; Wiśniewska, H.; Ochodzki, P.; Walentyn-Góral, D. Higher Fusarium Toxin Accumulation in Grain of Winter Triticale Lines Inoculated with Fusarium culmorum as Compared with Wheat. Toxins 2016, 8, 301. https://doi.org/10.3390/toxins8100301
Góral T, Wiśniewska H, Ochodzki P, Walentyn-Góral D. Higher Fusarium Toxin Accumulation in Grain of Winter Triticale Lines Inoculated with Fusarium culmorum as Compared with Wheat. Toxins. 2016; 8(10):301. https://doi.org/10.3390/toxins8100301
Chicago/Turabian StyleGóral, Tomasz, Halina Wiśniewska, Piotr Ochodzki, and Dorota Walentyn-Góral. 2016. "Higher Fusarium Toxin Accumulation in Grain of Winter Triticale Lines Inoculated with Fusarium culmorum as Compared with Wheat" Toxins 8, no. 10: 301. https://doi.org/10.3390/toxins8100301
APA StyleGóral, T., Wiśniewska, H., Ochodzki, P., & Walentyn-Góral, D. (2016). Higher Fusarium Toxin Accumulation in Grain of Winter Triticale Lines Inoculated with Fusarium culmorum as Compared with Wheat. Toxins, 8(10), 301. https://doi.org/10.3390/toxins8100301