Inhibitory Activities of Alkyl Syringates and Related Compounds on Aflatoxin Production
Abstract
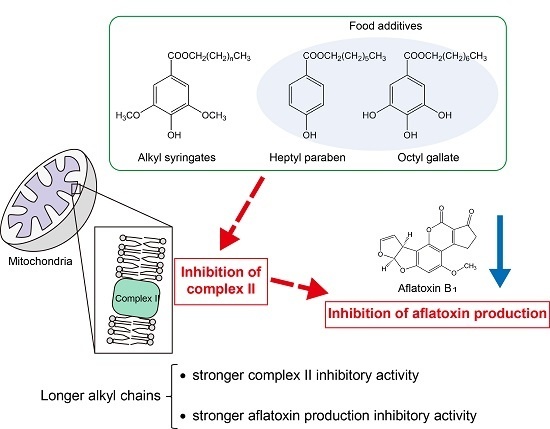
:1. Introduction
2. Results and Discussion
2.1. Aflatoxin Production Inhibitory Activity of Alkyl Syringates and Related Compounds
2.2. Inhibitory Activities of Alkyl Syringates on Mitochondrial Complex II
2.3. Effects of Octyl Syringate, Octyl Paraben, and Octyl Gallate on the mRNA Levels of Genes Encoding Proteins Responsible for Aflatoxin Biosynthesis
3. Conclusions
4. Experimental Section
4.1. Strains and Culture Conditions
4.2. Synthesis of Alkyl Syringates, and Octyl Paraben
4.3. Aflatoxin Analysis
4.4. Complex II Activity Analysis
4.5. RT-qPCR Analysis of the Genes Encoding Proteins Responsible for Aflatoxin Biosynthesis
4.6. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, C.C.H.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, J.; Takagi, K.; Iimura, K.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotech. Biochem. 2016, 80, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Prabowo, D.F.; Takagi, K.; Shiomi, K.; Mori, M.; Ōmura, S.; Nagasawa, H. Inhibitory effects of respiration inhibitors on aflatoxin production. Toxins 2014, 6, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Jermnak, U.; Yoshinari, T.; Sugiyama, Y.; Tsuyuki, R.; Nagasawa, H.; Sakuda, S. Isolation of methyl syringate as a specific aflatoxin production inhibitor from the essential oil of Betula alba and aflatoxin production inhibitory activities of its related compounds. Int. J. Food Microbiol. 2012, 153, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Nakagawa, Y.; Yazawa, S.; Sasaki, Y.; Yajima, S. Antifungal activity of alkyl gallates against plant pathogenic fungi. Bioorg. Med. Chem. Lett. 2014, 24, 1812–1814. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yazawa, S.; Nakagawa, Y.; Sasaki, Y.; Yajima, S. Effects of alkyl parabens on plant pathogenic fungi. Bioorg. Med. Chem. Lett. 2015, 25, 1774–1777. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Rodriguez, M.; Etcheverry, M. Control of Aspergillus growth and aflatoxin production using antioxidants at different conditions of water activity and pH. J. Appl. Microbiol. 2003, 95, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Leal, P.C.; Mascarello, A.; Derita, M.; Zuljan, F.; Nunes, R.J.; Zacchino, S.; Yunes, R.A. Relation between lipophilicity of alkyl gallates and antifungal activity against yeasts and filamentous fungi. Bioorg. Med. Chem. Lett. 2009, 19, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathways genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Akiyama, T.; Nakamura, K.; Kondo, T.; Takahashi, Y.; Muraoka, Y.; Nonomura, Y.; Nagasawa, H.; Sakuda, S. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology 2007, 153, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- Yoshinari, T.; Sakuda, S.; Watanabe, M.; Kamata, Y.; Ohnishi, T.; Sugita-Konishi, Y. A new metabolic pathway for converting blasticidin S in Aspergillus flavus and inhibitory activity of aflatoxin production by blasticidin S metabolites. J. Agric. Food Chem. 2013, 61, 7925–7931. [Google Scholar] [CrossRef] [PubMed]



| Compound | IC50 (mM) |
|---|---|
| methyl syringate (1) | - * |
| ethyl syringate (2) | - * |
| propyl syringate (3) | >20 |
| butyl syringate (4) | 9.7 |
| pentyl syringate (5) | 2.8 |
| hexyl syringate (6) | 1.1 |
| heptyl syringate (7) | 1.3 |
| octyl syringate (8) | 0.34 |
| propyl paraben (10) | >20 |
| octyl paraben (12) | 0.16 |
| octyl gallate (14) | 0.29 |
| boscalid | 0.019 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furukawa, T.; Iimura, K.; Kimura, T.; Yamamoto, T.; Sakuda, S. Inhibitory Activities of Alkyl Syringates and Related Compounds on Aflatoxin Production. Toxins 2016, 8, 177. https://doi.org/10.3390/toxins8060177
Furukawa T, Iimura K, Kimura T, Yamamoto T, Sakuda S. Inhibitory Activities of Alkyl Syringates and Related Compounds on Aflatoxin Production. Toxins. 2016; 8(6):177. https://doi.org/10.3390/toxins8060177
Chicago/Turabian StyleFurukawa, Tomohiro, Kurin Iimura, Taichi Kimura, Toshiyoshi Yamamoto, and Shohei Sakuda. 2016. "Inhibitory Activities of Alkyl Syringates and Related Compounds on Aflatoxin Production" Toxins 8, no. 6: 177. https://doi.org/10.3390/toxins8060177
APA StyleFurukawa, T., Iimura, K., Kimura, T., Yamamoto, T., & Sakuda, S. (2016). Inhibitory Activities of Alkyl Syringates and Related Compounds on Aflatoxin Production. Toxins, 8(6), 177. https://doi.org/10.3390/toxins8060177