The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus
Abstract
:1. Introduction
2. Results
2.1. Aspergillus Flavus Skn7 Sequence Analysis
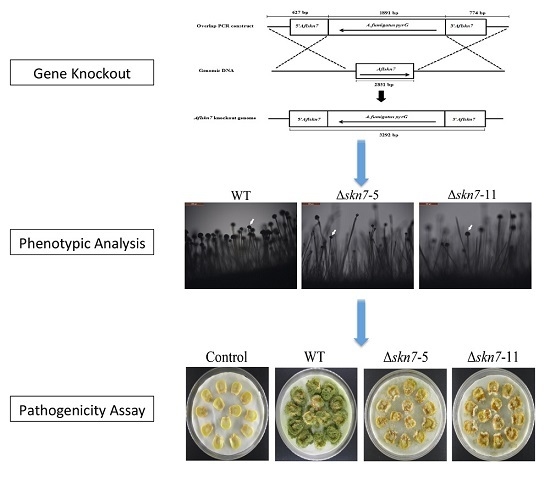
2.2. Construction of ΔAflSkn7 Deletion Mutants
2.3. Involvement of AflSkn7 during Asexual Development
2.4. Requirement of AflSkn7 during Sexual Development
2.5. Effects of the Deletion of AflSkn7 on Sensitivity to Osmotic and Cell Wall Stresses
2.6. Effects of the Deletion of AflSkn7 on Sensitivity to Oxidative Stress
2.7. Role of AflSkn7 for Aflatoxin Biosynthesis
2.8. Contribution of AflSkn7 to Pathogenicity
3. Discussion
4. Materials and Methods
4.1. Fungal Strains and Growth Conditions
4.2. Sequence Analysis
4.3. Gene Deletion
4.4. Molecular Manipulations
4.5. Stress Sensitivity Assay
4.6. Aflatoxin Analysis
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction Analysis
4.8. Pathogenicity Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amaike, S.; Keller, N.P. Aspergillus flavus. Annu. Rev. Phytopathol. 2011, 49, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Chang, P.-K.; Shams-Ghahfarokhi, M.; Rai, M. Global health issues of aflatoxins in food and agriculture: Challenges and opportunities. Front. Microbiol. 2014, 5, 420. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food. Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Grintzalis, K.; Vernardis, S.I.; Klapa, M.I.; Georgiou, C.D. Role of oxidative stress in Sclerotial differentiation and aflatoxin B1 biosynthesis in Aspergillus flavus. Appl. Environ. Microbiol. 2014, 80, 5561–5571. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Guo, Z.; Zhong, H.; Wang, S.; Yang, W.; Liu, Y.; Wang, S. RNA-Seq-based transcriptome analysis of aflatoxigenic Aspergillus flavus in response to water activity. Toxins 2014, 6, 3187–3207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhong, H.; Han, X.; Guo, Z.; Yang, W.; Liu, Y.; Yang, K.; Zhuang, Z.; Wang, S. Proteomic profile of Aspergillus flavus in response to water activity. Fungal Biol. 2015, 119, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, S.; Zhong, H.; Yang, Q.; Zhang, F.; Zhuang, Z.; Yuan, J.; Nie, X.; Wang, S. Integrative analyses reveal transcriptome-proteome correlation in biological pathways and secondary metabolism clusters in, A. flavus in response to temperature. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Fassler, J.S.; West, A.H. Fungal Skn7 stress responses and their relationship to virulence. Eukaryot. Cell 2011, 10, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, S.; Larkov, O.; Lamdan, N.L.; Horwitz, B.A. Genetic interaction of the stress response factors ChAP1 and Skn7 in the maize pathogen Cochliobolus heterostrophus. FEMS Microbiol. Lett. 2014, 350, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Mulford, K.; Fassler, J. Association of the Skn7 and Yap1 transcription factors in the Saccharomyces cerevisiae oxidative stress response. Eukaryot. Cell 2011, 10, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yin, D.; Yin, Y.; Cao, Y.; Ma, Z. The response regulator BcSkn7 is required for vegetative differentiation and adaptation to oxidative and osmotic stresses in Botrytis cinerea. Mol. Plant Pathol. 2015, 16, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Chen, P.; Chen, Y.; Lu, Y.; Wang, C. MrSkn7 Controls Sporulation, Cell Wall Integrity, Autolysis, and Virulence in Metarhizium robertsii. Eukaryot. Cell 2015, 14, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Roze, L.V.; Linz, J.E. Oxidative stress-related transcription factors in the regulation of secondary metabolism. Toxins 2013, 5, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Pérez, I.; Sánchez, O.; Kawasaki, L.; Georgellis, D.; Aguirre, J. Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot. Cell 2007, 6, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, D.; Mizuno, T.; Abe, K. Characterization of the conserved phosphorylation site in the Aspergillus nidulans response regulator SrrA. Curr. Genet. 2011, 57, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Lin, C.-H.; Chung, K.-R. Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet. Biol. 2012, 49, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Lamarre, C.; Ibrahim-Granet, O.; Du, C.; Calderone, R.; Latgé, J.-P. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet. Biol. 2007, 44, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, S.; Zhang, Q.; Tao, Y.; Wang, C.; Xu, J.R. FgSKN7 and FgATF1 have overlapping functions in ascosporogenesis, pathogenesis and stress responses in Fusarium graminearum. Environ. Microbiol. 2015, 17, 1245–1260. [Google Scholar] [CrossRef] [PubMed]
- Bahn, Y.-S.; Kojima, K.; Cox, G.M.; Heitman, J. A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol. Biol. Cell 2006, 17, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Viefhues, A.; Schlathoelter, I.; Simon, A.; Viaud, M.; Tudzynski, P. Unraveling the function of the response regulator BcSkn7 in the stress signaling network of Botrytis cinerea. Eukaryot. Cell 2015, 14, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, R.; Yamada, H.; Kato, C.; Aiba, H.; Mizuno, T. The Prr1 response regulator is essential for transcription of ste11+ and for sexual development in fission yeast. Mol. Gen. Genet. 2000, 264, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Requena, N.; Fischer, R. The MAPKK kinase SteC regulates conidiophore morphology and is essential for heterokaryon formation and sexual development in the homothallic fungus Aspergillus nidulans. Mol. Microbiol. 2003, 47, 1577–1588. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.A.; Banks, G.R.; Toone, W.M.; Raitt, D.; Kuge, S.; Johnston, L.H. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997, 16, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Ochiai, N.; Morita, M.; Iida, Y.; Usami, R.; Kudo, T. Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr. Genet. 2008, 54, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 2011, 189, 1145–1175. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Laivenieks, M.; Hong, S.Y.; Wee, J.; Wong, S.S.; Vanos, B.; Awad, D.; Ehrlich, K.C.; Linz, J.E. Aflatoxin biosynthesis is a novel source of reactive oxygen species—A potential redox signal to initiate resistance to oxidative stress? Toxins 2015, 7, 1411–1430. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Zjalic, S.; Ricelli, A.; Punelli, F.; Camera, E.; Fabbri, C.; Picardo, M.; Fanelli, C.; Fabbri, A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: A role for the ApyapA gene. Eukaryot. Cell 2008, 7, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, M.; Gazzetti, K.; Punelli, F.; Scarpari, M.; Zjalic, S.; Ricelli, A.; Fabbri, A.A.; Fanelli, C. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl. Microbial. Biotechnol. 2012, 95, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Roze, L.V.; Wee, J.; Linz, J.E. Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in aspergilli. Microbiologyopen 2013, 2, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Aspergillus Genome Projects. Available online: http://www.broadinstitute.org/annotation/genome (accessed on 12 October 2013).
- Simple Modular Architecture Research Tool. Available online: http://smart.embl-heidelberg.de/ (accessed on 6 November 2013).
- Illustrator for Biological Sequences program. Available online: http://ibs.biocuckoo.org/ (accessed on 2 December 2013).
- Szewczyk, E.; Nayak, T.; Oakley, C.E.; Edgerton, H.; Xiong, Y.; Taheri-Talesh, N.; Osmani, S.A.; Oakley, B.R. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 2006, 1, 3111–3120. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Brodhagen, M.; Isakeit, T.; Brown, S.H.; Göbel, C.; Betran, J.; Feussner, I.; Keller, N.P.; Kolomiets, M.V. Inactivation of the lipoxygenase ZmLOX3 increases susceptibility of maize to Aspergillus spp. Mol. Plant Microbe Interact. 2009, 22, 222–231. [Google Scholar] [CrossRef] [PubMed]










© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Xu, G.; Geng, L.; Lu, X.; Yang, K.; Yuan, J.; Nie, X.; Zhuang, Z.; Wang, S. The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus. Toxins 2016, 8, 202. https://doi.org/10.3390/toxins8070202
Zhang F, Xu G, Geng L, Lu X, Yang K, Yuan J, Nie X, Zhuang Z, Wang S. The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus. Toxins. 2016; 8(7):202. https://doi.org/10.3390/toxins8070202
Chicago/Turabian StyleZhang, Feng, Gaopo Xu, Longpo Geng, Xiaoyan Lu, Kunlong Yang, Jun Yuan, Xinyi Nie, Zhenhong Zhuang, and Shihua Wang. 2016. "The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus" Toxins 8, no. 7: 202. https://doi.org/10.3390/toxins8070202
APA StyleZhang, F., Xu, G., Geng, L., Lu, X., Yang, K., Yuan, J., Nie, X., Zhuang, Z., & Wang, S. (2016). The Stress Response Regulator AflSkn7 Influences Morphological Development, Stress Response, and Pathogenicity in the Fungus Aspergillus flavus. Toxins, 8(7), 202. https://doi.org/10.3390/toxins8070202