Cells Deficient in the Fanconi Anemia Protein FANCD2 are Hypersensitive to the Cytotoxicity and DNA Damage Induced by Coffee and Caffeic Acid
Abstract
:1. Introduction
2. Results
2.1. Cells Deficient in FANCD2 Are Hypersensitive to the Cytotoxicity of Coffee and Caffeic Acid
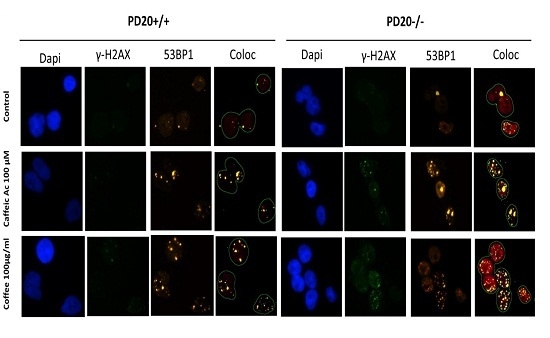
2.2. Cells Deficient in FANCD2 Are Hypersensitive to the DNA Damage Induced by Coffee and Caffeic Acid
3. Discussion
4. Materials and Methods
4.1. Chemicals and Cell Lines
4.2. Clonogenic Assay
4.3. Immunofluorescence γ-H2AX and 53BP1 Focus Assay
Author Contributions
Conflicts of Interest
Abbreviations
| ALL | acute lymphoblastic leukemia |
| AML | acute myeloid leukemia |
| DSBs | double strand breaks |
| FA | Fanconi anemia |
| IARC | International Agency for Research on Cancer |
| ROS | reactive oxygen species |
References
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and potential impact on health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Frei, B. Coffee and health: A review of recent human research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Satija, A.; Bhupathiraju, S.N.; Hu, Y.; Sun, Q.; Han, J.; Lopez-Garcia, E.; Willett, W.; van Dam, R.M.; Hu, F.B. Association of Coffee Consumption with Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation 2015, 132, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Bohn, S.K.; Blomhoff, R.; Paur, I. Coffee and cancer risk, epidemiological evidence, and molecular mechanisms. Mol. Nutr. Food Res. 2014, 58, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, K.; Sugawara, Y.; Tomata, Y.; Nishino, Y.; Fukao, A.; Tsuji, I. The association between coffee consumption and bladder cancer incidence in a pooled analysis of the Miyagi Cohort Study and Ohsaki Cohort Study. Eur. J. Cancer Prev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Arab, L. Epidemiologic evidence on coffee and cancer. Nutr. Cancer 2010, 62, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tong, Y.; Zhao, Q.; Yu, G.; Wei, X.; Lu, Q. Coffee consumption and bladder cancer: A meta-analysis of observational studies. Sci. Rep. 2015, 5, 9051. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, A.; Rudant, J.; Goujon-Bellec, S.; Orsi, L.; Leverger, G.; Baruchel, A.; Bertrand, Y.; Nelken, B.; Pasquet, M.; Michel, G.; et al. Childhood acute leukemia, maternal beverage intake during pregnancy, and metabolic polymorphisms. Cancer Causes Control 2013, 24, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, T.P.; Ntouvelis, E.; Diamantaras, A.A.; Tzanoudaki, M.; Baka, M.; Hatzipantelis, E.; Kourti, M.; Polychronopoulou, S.; Sidi, V.; Stiakaki, E.; et al. Maternal and childhood consumption of coffee, tea and cola beverages in association with childhood leukemia: A meta-analysis. Cancer Epidemiol. 2015, 39, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Su, H.; Zhu, R.; Wang, X.; Peng, M.; Song, J.; Fan, D. Maternal coffee consumption during pregnancy and risk of childhood acute leukemia: A metaanalysis. Am. J. Obstet. Gynecol. 2014, 210, 151. [Google Scholar] [CrossRef] [PubMed]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control 2015, 26, 1003–1017. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic acids from green coffee extract are highly bioavailable in humans. J. Nutr. 2008, 138, 2309–2015. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic acids and other cinnamates – nature, occurrence and dietary burden. J. Sci.Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, A.H.; Perks, C.M.; Zeng, L.; Markkula, A.; Simonsson, M.; Rose, C.; Ingvar, C.; Holly, J.M.; Jernstrom, H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Schipp, D.; Galan, J.; Eisenbrand, G.; Richling, E. Coffee consumption rapidly reduces background DNA strand breaks in healthy humans: Results of a short term repeated uptake intervention study. Mol. Nutr. Food Res. 2016, 60, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Sultana, S. Attenuation of oxidative stress, inflammation and early markers of tumor promotion by caffeic acid in Fe-NTA exposed kidneys of Wistar rats. Mol. Cell. Biochem. 2011, 357, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and kidney carcinogenicity of caffeic acid in F344 rats and C57BL/6Nx C3H/HeN F1 mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar] [PubMed]
- Szeto, Y.T.; Collins, A.R.; Benzie, I.F. Effects of dietary antioxidants on DNA damage in lysed cells using a modified comet assay procedure. Mutat. Res. 2002, 500, 31–38. [Google Scholar] [CrossRef]
- Bhat, S.H.; Azmi, A.S.; Hadi, S.M. Prooxidant DNA breakage induced by caffeic acid in human peripheral lymphocytes: Involvement of endogenous copper and a putative mechanism for anticancer properties. Toxicol. Appl. Pharmacol. 2007, 218, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Maistro, E.L.; Angeli, J.P.; Andrade, S.F.; Mantovani, M.S. In vitro genotoxicity assessment of caffeic, cinnamic and ferulic acids. Genet. Mol. Res. 2011, 10, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Ito, K.; Yamamoto, K.; Kawanishi, S. Caffeic acid causes metal-dependent damage to cellular and isolated DNA through H2O2 formation. Carcinogenesis 1992, 13, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Trush, M.A. Reactive oxygen-dependent DNA damage resulting from the oxidation of phenolic compounds by a copper-redox cycle mechanism. Cancer Res. 1994, 54, 1895s–1898s. [Google Scholar] [PubMed]
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research strategies in the study of the pro-oxidant nature of polyphenol nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G. Cancer genetics. Am. J. Med. Genet. 2002, 111, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Greenman, C.; Stephens, P.; Smith, R.; Dalgliesh, G.L.; Hunter, C.; Bignell, G.; Davies, H.; Teague, J.; Butler, A.; Stevens, C.; et al. Patterns of somatic mutation in human cancer genomes. Nature 2007, 446, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Burrell, R.A.; McGranahan, N.; Bartek, J.; Swanton, C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature 2013, 501, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Errol, C.F.; Walker, G.C.; Siede, W. DNA Repair and Mutagenesis; ASM Press: Washington, DC, USA, 1995. [Google Scholar]
- D’Andrea, A.D.; Grompe, M. Molecular biology of Fanconi anemia: Implications for diagnosis and therapy. Blood 1997, 90, 1725–1736. [Google Scholar] [PubMed]
- Willers, H.; Kachnic, L.A.; Luo, C.M.; Li, L.; Purschke, M.; Borgmann, K.; Held, K.D.; Powell, S.N. Biomarkers and mechanisms of FANCD2 function. J. Biomed. Biotechnol. 2008, 2008, 821529. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, J.; Pickering, A.; Zhang, J.; Wang, H.; Tian, H.; Zheng, J.; Fei, P. A hidden role of the inactivated FANCD2: Upregulating DeltaNp63. Oncotarget 2013, 4, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, D.; Park, H.K.; Wang, H.; Dyer, R.B.; Liu, W.; Klee, G.G.; McNiven, M.A.; Tindall, D.J.; Molina, J.R.; et al. FAVL elevation in human tumors disrupts Fanconi anemia pathway signaling and promotes genomic instability and tumor growth. J. Clin. Investig. 2010, 120, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Panneerselvam, J.; Park, H.K.; Zhang, J.; Dudimah, F.D.; Zhang, P.; Wang, H.; Fei, P. FAVL impairment of the Fanconi anemia pathway promotes the development of human bladder cancer. Cell Cycle 2012, 11, 2947–2455. [Google Scholar] [CrossRef] [PubMed]
- Tischkowitz, M.D.; Morgan, N.V.; Grimwade, D.; Eddy, C.; Ball, S.; Vorechovsky, I.; Langabeer, S.; Stoger, R.; Hodgson, S.V.; Mathew, C.G. Deletion and reduced expression of the Fanconi anemia FANCA gene in sporadic acute myeloid leukemia. Leukemia 2004, 18, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Joenje, H.; Arwert, F.; Eriksson, A.W.; de Koning, H.; Oostra, A.B. Oxygen-dependence of chromosomal aberrations in Fanconi’s anaemia. Nature 1981, 290, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Hammond, A.T.; Moses, R.E. Hypersensitivity to oxygen is a uniform and secondary defect in Fanconi anemia cells. Mutat. Res. 1993, 294, 255–262. [Google Scholar] [CrossRef]
- Takeuchi, T.; Morimoto, K. Increased formation of 8-hydroxydeoxyguanosine, an oxidative DNA damage, in lymphoblasts from Fanconi’s anemia patients due to possible catalase deficiency. Carcinogenesis 1993, 14, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.L.; Baptista, T.; Amado, F.; Vitorino, R.; Jeronimo, C.; Helguero, L.A. Expression and functionality of histone H2A variants in cancer. Oncotarget 2014, 5, 3428–4343. [Google Scholar] [CrossRef] [PubMed]
- Wittemer, S.M.; Ploch, M.; Windeck, T.; Muller, S.C.; Drewelow, B.; Derendorf, H.; Veit, M. Bioavailability and pharmacokinetics of caffeoylquinic acids and flavonoids after oral administration of Artichoke leaf extracts in humans. Phytomedicine 2005, 12, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Guy, P.A.; Marmet, C.; Fraering, A.L.; Longet, K.; Moulin, J.; Enslen, M.; Barron, D.; Dionisi, F.; Cavin, C.; et al. Measurement of caffeic and ferulic acid equivalents in plasma after coffee consumption: Small intestine and colon are key sites for coffee metabolism. Mol. Nutr. Food Res. 2010, 54, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Orta, M.L.; Pastor, N.; Perez-Guerrero, C.; Austin, C.; Mateos, S.; Lopez-Lazaro, M. The Coffee Constituent Chlorogenic Acid Induces Cellular DNA Damage and Formation of Topoisomerase I- and II-DNA Complexes in Cells. J. Agric. Food Chem. 2012, 60, 7384–7391. [Google Scholar] [CrossRef] [PubMed]
- Long, L.H.; Halliwell, B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human volunteers. Free Radic. Res. 2000, 32, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Kida, T.; Kikugawa, K. Increased urinary hydrogen peroxide levels caused by coffee drinking. Biol. Pharm. Bull. 2002, 25, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Long, L.H.; Yee, T.P.; Lim, S.; Kelly, R. Establishing biomarkers of oxidative stress: The measurement of hydrogen peroxide in human urine. Curr. Med. Chem. 2004, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Dual role of hydrogen peroxide in cancer: Possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007, 252, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, M.; Shigemitsu, T.; Suyama, K. Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 2003, 67, 2632–2640. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Orta, M.L.; Calderon-Montano, J.; Dominguez, I.; Pastor, N.; Burgos-Moron, E.; Lopez-Lazaro, M.; Cortes, F.; Mateos, S.; Helleday, T. 5-Aza-2′-deoxycytidine causes replication lesions that require Fanconi anemia-dependent homologous recombination for repair. Nucleic Acids Res. 2013, 41, 5827–5836. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgos-Morón, E.; Calderón-Montaño, J.M.; Orta, M.L.; Guillén-Mancina, E.; Mateos, S.; López-Lázaro, M. Cells Deficient in the Fanconi Anemia Protein FANCD2 are Hypersensitive to the Cytotoxicity and DNA Damage Induced by Coffee and Caffeic Acid. Toxins 2016, 8, 211. https://doi.org/10.3390/toxins8070211
Burgos-Morón E, Calderón-Montaño JM, Orta ML, Guillén-Mancina E, Mateos S, López-Lázaro M. Cells Deficient in the Fanconi Anemia Protein FANCD2 are Hypersensitive to the Cytotoxicity and DNA Damage Induced by Coffee and Caffeic Acid. Toxins. 2016; 8(7):211. https://doi.org/10.3390/toxins8070211
Chicago/Turabian StyleBurgos-Morón, Estefanía, José Manuel Calderón-Montaño, Manuel Luis Orta, Emilio Guillén-Mancina, Santiago Mateos, and Miguel López-Lázaro. 2016. "Cells Deficient in the Fanconi Anemia Protein FANCD2 are Hypersensitive to the Cytotoxicity and DNA Damage Induced by Coffee and Caffeic Acid" Toxins 8, no. 7: 211. https://doi.org/10.3390/toxins8070211
APA StyleBurgos-Morón, E., Calderón-Montaño, J. M., Orta, M. L., Guillén-Mancina, E., Mateos, S., & López-Lázaro, M. (2016). Cells Deficient in the Fanconi Anemia Protein FANCD2 are Hypersensitive to the Cytotoxicity and DNA Damage Induced by Coffee and Caffeic Acid. Toxins, 8(7), 211. https://doi.org/10.3390/toxins8070211