Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus
Abstract
:1. Introduction
2. Results
2.1. Effect of Aqueous Extract of Hyssop on the Production of AFB1 and the Development of A. flavus
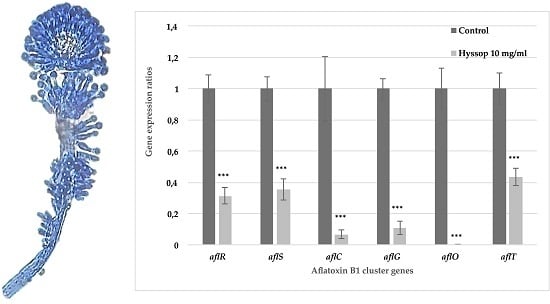
2.2. Aqueous Extract of Hyssop Down-Regulated the Expression of AFB1 Cluster Genes
2.3. Transcriptomic Effect of Hyssop Extract on the Expression of Genes Coding for Regulators of Secondary Metabolites
3. Discussion
3.1. Hyssop Leads to an Inhibition of AFB1 Synthesis in A. flavus by a Transcriptomic Regulation of AFB1 Cluster Genes
3.2. The Implication of VeA and MtfA: Two Leading Transcriptional Regulators
3.3. Implication of Other Regulatory Factors in AF Inhibition by M. graeca—Hyssop Extract
3.4. Morphological Modifications of Conidiophores and Vesicles of A. flavus in Hyssop-Supplemented Media
4. Conclusions
5. Materials and Methods
5.1. Solvents and Standards
5.2. Preparation of the Aqueous Solution of Hyssop
5.3. Fungal Strains and Growth Conditions
5.4. Examination of Cultural Parameters
5.4.1. Effect on Growth
5.4.2. Mycelium Dry Weight
5.4.3. Total Spore Quantification
5.4.4. Delay to Germination
5.4.5. Fungal Morphological Features
5.5. RNA Isolation and Reverse Transcription
5.6. Real-Time PCR Expression Profile Analysis of Genes Regulating AFB1 Biosynthesis in A. flavus
5.7. Aflatoxin Extraction and HPLC Quantification
5.8. Statistics
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Cano, P.; Puel, O.; Oswald, I.P. Mycotoxins: Fungal Secondary Metabolites with Toxic Properties. In Fungi Applications and Management Strategies; Deshmukh, S.K., Misra, J.K., Tewari, J.P., Papp, T., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 318–371. [Google Scholar]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Wu, F.; Guclu, H. Aflatoxin regulations in a network of global maize trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of climate change on Aspergillus flavus and aflatoxin B1 production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Cruz Cabral, L.; Fernández Pinto, V.; Patriarca, A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013, 166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; He, B.; Zhang, L.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. A Structure identification and toxicity assessment of the degradation products of aflatoxin B1 in peanut oil under UV irradiation. Toxins (Basel) 2016, 8, 332. [Google Scholar] [CrossRef] [PubMed]
- Diao, E.; Li, X.; Zhang, Z.; Ma, W.; Ji, N.; Dong, H. Ultraviolet irradiation detoxification of aflatoxins. Trends Food Sci. Technol. 2015, 42, 64–69. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Q.; Ma, S.; Zhang, J.; Jia, R.; Ji, C.; Zhao, L. Ameliorating effects of Bacillus subtilis ANSB060 on growth performance, antioxidant functions, and aflatoxin residues in ducks fed diets contaminated with aflatoxins. Toxins (Basel) 2016, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.-Y.; Qi, M.; Zhao, L.; Zhu, M.-K.; Guo, J.; Liu, J.; Gu, C.-Q.; Rajput, S.; Krumm, C.; Qi, D.-S.; et al. Curcumin prevents aflatoxin B1 hepatoxicity by inhibition of cytochrome P450 isozymes in chick liver. Toxins (Basel) 2016, 8, 327. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Eisa Ahmed, M.; Sangare, L.; Zhao, Y.; Selvaraj, J.; Xing, F.; Wang, Y.; Yang, H.; Liu, Y. Novel aflatoxin-degrading enzyme from Bacillus shackletonii L7. Toxins (Basel) 2017, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Dellafiora, L.; Galaverna, G.; Reverberi, M.; Dall’Asta, C. Degradation of aflatoxins by means of laccases from Trametes versicolor: An in silico insight. Toxins (Basel) 2017, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Saladino, F.; Luz, C.; Manyes, L.; Fernández-Franzón, M.; Meca, G. In vitro antifungal activity of lactic acid bacteria against mycotoxigenic fungi and their application in loaf bread shelf life improvement. Food Control 2016, 67, 273–277. [Google Scholar] [CrossRef]
- Mauro, A.; Battilani, P.; Cotty, P.J. Atoxigenic Aspergillus flavus endemic to Italy for biocontrol of aflatoxins in maize. Biocontrol 2015, 60, 125–134. [Google Scholar] [CrossRef]
- Odhiambo, B.O.; Murage, H.; Wagara, I.N. Screening for Atoxigenic Aspergillus Species and Evaluating their Inhibitory Potential against Growth and Sporulation of Aflatoxigenic Aspergillus Species. Egert. J. Sci. Technol. 2014, 14, 61–80. [Google Scholar]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Jane, C.; Kiprop, E.K.; Mwamburi, L.A. Biocontrol of Aflatoxins in Corn using Atoxigenic Aspergillus flavus: Review. Int. J. Sci. Res. 2014, 3, 1954–1958. [Google Scholar]
- Kohiyama, C.Y.; Yamamoto Ribeiro, M.M.; Mossini, S.A.G.; Bando, E.; Bomfim, N.D.S.; Nerilo, S.B.; Rocha, G.H.O.; Grespan, R.; Mikcha, J.M.G.; Machinski, M. Antifungal properties and inhibitory effects upon aflatoxin production of Thymus vulgaris L. by Aspergillus flavus Link. Food Chem. 2015, 173, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Chulze, S.N. Strategies to reduce mycotoxin levels in maize during storage: A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Abu-Gharbieh, E.; Shehab, N.G.; Khan, S.A. Anti-inflammatory and gastroprotective activities of the aqueous extract of Micromeria fruticosa (L.) Druce ssp Serpyllifolia in mice. Pak. J. Pharm. Sci. 2013, 26, 799–803. [Google Scholar] [PubMed]
- Džamić, A.M.; Soković, M.D.; Novaković, M.; Jadranin, M.; Ristić, M.S.; Tešević, V.; Marin, P.D. Composition, antifungal and antioxidant properties of Hyssopus officinalis L. subsp. pilifer (Pant.) Murb. essential oil and deodorized extracts. Ind. Crops Prod. 2013, 51, 401–407. [Google Scholar] [CrossRef]
- Michalczyk, M.; Macura, R.; Tesarowicz, I.; Banaś, J. Effect of adding essential oils of coriander (Coriandrum sativum L.) and hyssop (Hyssopus officinalis L.) on the shelf life of ground beef. Meat Sci. 2012, 90, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Kurt, S.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tisserand, R. Essential oil safety II. metabolism, neurotoxicity, reproductive toxicity. Int. J. Aromather. 1996, 7, 26–29. [Google Scholar] [CrossRef]
- Alwan, S.; El Omari, K.; Soufi, H.; Zreika, S.; Sukarieh, I.; Chihib, N.-E.; Jama, C.; Hamze, M. Evaluation of the antibacterial activity of Micromeria barbata in Lebanon. J. Essent. Oil Bear. Plants 2016, 19, 321–327. [Google Scholar] [CrossRef]
- Formisano, C.; Oliviero, F.; Rigano, D.; Saab, A.M.; Senatore, F. Chemical composition of essential oils and in vitro antioxidant properties of extracts and essential oils of Calamintha origanifolia and Micromeria myrtifolia, two Lamiaceae from the Lebanon flora. Ind. Crops Prod. 2014, 62, 405–411. [Google Scholar] [CrossRef]
- Skotti, E.; Anastasaki, E.; Kanellou, G.; Polissiou, M.; Tarantilis, P.A. Total phenolic content, antioxidant activity and toxicity of aqueous extracts from selected Greek medicinal and aromatic plants. Ind. Crops Prod. 2014, 53, 46–54. [Google Scholar] [CrossRef]
- Chang, P.-K.; Yu, J.; Yu, J.-H. aflT, a MFS transporter-encoding gene located in the aflatoxin gene cluster, does not have a significant role in aflatoxin secretion. Fungal Genet. Biol. 2004, 41, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Caceres, I.; El Khoury, R.; Medina, Á.; Lippi, Y.; Naylies, C.; Atoui, A.; El Khoury, A.; Oswald, I.P.; Bailly, J.-D.; Puel, O. Deciphering the anti-aflatoxinogenic properties of eugenol using a large-scale q-PCR approach. Toxins (Basel) 2016, 8, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omidpanah, S.; Sadeghi, H.; Mohamadian, M.; Manayi, A. Evaluation of antifungal activity of aqueous extracts of some medicinal plants against Aspergillus flavus, pistachio aflatoxin producing fungus in vitro. Drug Dev. Ther. 2015, 6, 66–69. [Google Scholar]
- Georgianna, D.R.; Payne, G.A. Genetic regulation of aflatoxin biosynthesis: From gene to genome. Fungal Genet. Biol. 2009, 46, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chang, P.-K.; Ehrlich, K.C.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C. Predicted roles of the uncharacterized clustered genes in aflatoxin biosynthesis. Toxins (Basel) 2009, 1, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Yu, J.; Cotty, P.J. Aflatoxin biosynthesis gene clusters and flanking regions. J. Appl. Microbiol. 2005, 99, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.A.; Boston, R.S.; Payne, G.A. Diverse inhibitors of aflatoxin biosynthesis. Appl. Microbiol. Biotechnol. 2008, 78, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Calvo, A.M. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 2008, 45, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Braus, G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.-J.; Keller, N.P.; Yu, J.-H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Baidya, S.; Duran, R.M.; Lohmar, J.M.; Harris-Coward, P.Y.; Cary, J.W.; Hong, S.-Y.; Roze, L.V.; Linz, J.E.; Calvo, A.M. VeA is associated with the response to oxidative stress in the aflatoxin producer Aspergillus flavus. Eukaryot. Cell 2014, 13, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Duran, R.M.; Cary, J.W.; Calvo, A.M. Production of cyclopiazonic acid, aflatrem, and aflatoxin by Aspergillus flavus is regulated by veA, a gene necessary for sclerotial formation. Appl. Microbiol. Biotechnol. 2007, 73, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Sprote, P.; Brakhage, A.A. The light-dependent regulator velvet A of Aspergillus nidulans acts as a repressor of the penicillin biosynthesis. Arch. Microbiol 2007, 188, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, V.; Dhingra, S.; Kincaid, A.; Shantappa, S.; Feng, X.; Calvo, A.M. The putative C2H2 transcription factor MtfA is a novel regulator of secondary metabolism and morphogenesis in Aspergillus nidulans. PLoS ONE 2013, 8, e74122. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Lohmar, J.M.; Satterlee, T.; Cary, J.W.; Calvo, A.M. The master transcription factor mtfA governs aflatoxin production, morphological development and pathogenicity in the fungus Aspergillus flavus. Toxins (Basel) 2016, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, T.; Subramanyam, C. Oxidative stress as a prerequisite for aflatoxin production by Aspergillus parasiticus. Free Radic. Biol. Med. 2000, 29, 981–985. [Google Scholar] [CrossRef]
- Jayashree, T.; Subramanyam, C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett. Appl. Microbiol. 1999, 28, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Roze, L.V.; Linz, J.E. Oxidative stress-related transcription factors in the regulation of secondary metabolism. Toxins (Basel) 2013, 5, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.-K.P.; Scharfenstein, L.L.L.; Luo, M.; Mahoney, N.; Molyneux, R.J.; Yu, J.; Brown, R.L.; Campbell, B.C. Loss of msnA, a putative stress regulatory gene, in Aspergillus parasiticus and Aspergillus flavus increased production of conidia, aflatoxins and kojic acid. Toxins (Basel) 2011, 3, 82–104. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Roze, L.V.; Wee, J.; Linz, J.E. Evidence that a transcription factor regulatory network coordinates oxidative stress response and secondary metabolism in aspergilli. Microbiologyopen 2013, 2, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Ke, R.; Haynes, K.; Stark, J. Modelling the activation of alkaline pH response transcription factor PacC in Aspergillus nidulans: Involvement of a negative feedback loop. J. Theor. Biol. 2013, 326, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Cary, J.W.; Harris-Coward, P.Y.; Ehrlich, K.C.; Mack, B.M.; Kale, S.P.; Larey, C.; Calvo, A.M. NsdC and NsdD affect Aspergillus flavus morphogenesis and aflatoxin production. Eukaryot. Cell 2012, 11, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.K.; Mack, B.M.; Wei, Q.; Bland, J.M.; Bhatnagar, D.; Cary, J.W. RNA sequencing of an nsdC mutant reveals global regulation of secondary metabolic gene clusters in Aspergillus flavus. Microbiol. Res. 2015, 182, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Liu, X.; Yin, Y.; Ma, Z. Involvement of a velvet protein FgVeA in the regulation of asexual development, lipid and secondary metabolisms and virulence in Fusarium graminearum. PLoS ONE 2011, 6, e28291. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.S.; Greene-Mcdowelle, D.M.; Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. Effects of volatile aldehydes from Aspergillus-resistant varieties of corn on Aspergillus parasiticus growth and aflatoxin biosynthesis. Toxicon 2000, 38, 1215–1223. [Google Scholar] [CrossRef]
- Yang, Q.; Borkovich, K.A. Mutational activation of a Gα(i) causes uncontrolled proliferation of aerial hyphae and increased sensitivity to heat and oxidative stress in Neurospora crassa. Genetics 1999, 151, 107–117. [Google Scholar] [PubMed]
- Han, K.H.; Seo, J.A.; Yu, J.H. Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attentuation of GanB (Gα) signalling. Mol. Microbiol. 2004, 53, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Affeldt, K.; Carrig, J.; Amare, M.G.; Keller, N. Global survey of canonical Aspergillus flavus G protein-coupled receptors. MBio 2014, 5, 1501–1514. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Keller, N.P. Signalling pathways connecting mycotoxin production and sporulation. Mol. Plant Pathol. 2006, 7, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Atoui, A.K.; Mansouri, A.; Boskou, G.; Kefalas, P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005, 89, 27–36. [Google Scholar] [CrossRef]
- EL Mahgubi, A.; Bailly, S.; Tadrist, S.; Querin, A.; Ouadia, A.; Oswald, I.P.; Bailly, J.-D. Distribution and toxigenicity of Aspergillus section Flavi in spices marketed in Morocco. Food Control 2013, 32, 143–148. [Google Scholar] [CrossRef]
- Tannous, J.; El Khoury, R.; Snini, S.P.; Lippi, Y.; El Khoury, A.; Atoui, A.; Lteif, R.; Oswald, I.P.; Puel, O. Sequencing, physical organization and kinetic expression of the patulin biosynthetic gene cluster from Penicillium expansum. Int. J. Food Microbiol. 2014, 189, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Huang, X.; Min, S. Rapid determination of aflatoxins in corn and peanuts. J. Chromatogr. A 2008, 1209, 271–274. [Google Scholar] [CrossRef] [PubMed]





| Observed Parameters | MEA | MEA + Hyssop 10 mg/mL | |
|---|---|---|---|
| Growth | Colony diameter (cm) | 4.25 ± 0.03 | 4.4 ± 0.03 |
| Mycelium dry weight (g) | 0.16 ± 0.03 | 0.15 ± 0.02 | |
| Sporulation | Germinating conidia after 16 h (%) | 96.5 ± 8.5% | 101.5 ± 4% |
| Total spore count | 8.1 × 108 ± 4.5 × 107 | 1.1 × 109 ± 9.9 × 107 | |
| Spore density (conidia/cm2) | 5.7 × 107 ± 2.6 × 106 | 7 × 107 ± 5.6 × 106 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Khoury, R.; Caceres, I.; Puel, O.; Bailly, S.; Atoui, A.; Oswald, I.P.; El Khoury, A.; Bailly, J.-D. Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins 2017, 9, 87. https://doi.org/10.3390/toxins9030087
El Khoury R, Caceres I, Puel O, Bailly S, Atoui A, Oswald IP, El Khoury A, Bailly J-D. Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins. 2017; 9(3):87. https://doi.org/10.3390/toxins9030087
Chicago/Turabian StyleEl Khoury, Rhoda, Isaura Caceres, Olivier Puel, Sylviane Bailly, Ali Atoui, Isabelle P. Oswald, André El Khoury, and Jean-Denis Bailly. 2017. "Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus" Toxins 9, no. 3: 87. https://doi.org/10.3390/toxins9030087
APA StyleEl Khoury, R., Caceres, I., Puel, O., Bailly, S., Atoui, A., Oswald, I. P., El Khoury, A., & Bailly, J. -D. (2017). Identification of the Anti-Aflatoxinogenic Activity of Micromeria graeca and Elucidation of Its Molecular Mechanism in Aspergillus flavus. Toxins, 9(3), 87. https://doi.org/10.3390/toxins9030087