Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species
Abstract
:1. Introduction
2. Results
2.1. Treatment of Cover Glasses
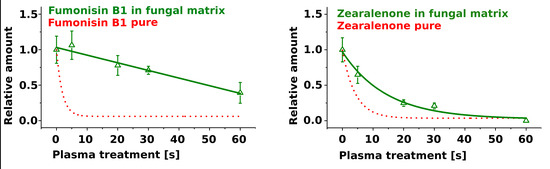
2.2. Treatment of Pure Mycotoxins and Fungal Extracts
3. Discussion
4. Experimental Setup and Materials
4.1. Mycotoxin Standards
4.2. Fungal Strains, Rice Cultures and Mycotoxin-Containing Fungal Extracts
4.3. Sample Preparation
4.4. Mycotoxin Analyses
4.5. Plasma Device
4.6. Measurement of Injected Power
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- He, J.; Zhou, T.; Young, J.C.; Boland, G.J.; Scott, P.M. Chemical and biological transformations for detoxification of trichothecene mycotoxins in human and animal food chains: A review. Trends Food Sci. Technol. 2010, 21, 67–76. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; de Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Helmke, A.; Hoffmeister, D.; Berge, F.; Emmert, S.; Laspe, P.; Mertens, N.; Vioel, W.; Weltmann, K.-D. Physical and microbiological characterisation of Staphylococcus epidermidis inactivation by dielectric barrier discharge plasma. Plasma Process. Polym. 2011, 8, 278–286. [Google Scholar] [CrossRef]
- Ehlbeck, J.; Schnabel, U.; Polak, M.; Winter, J.; von Woedtke, T.; Brandenburg, R.; von dem Hagen, T.; Weltmann, K.-D. Low temperature atmospheric pressure plasma sources for microbial decontamination. J. Phys. D Appl. Phys. 2011, 44. [Google Scholar] [CrossRef]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied Plasma Medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Kong, M.G.; Kroesen, G.; Morfill, G.; Nosenko, T.; Shimizu, T.; van Dijk, J.; Zimmermann, J.L. Plasma medicine: An introductory review. New J. Phys. 2009, 11. [Google Scholar] [CrossRef]
- Park, G.Y.; Park, S.J.; Choi, M.Y.; Koo, I.G.; Byun, J.H.; Hong, J.W.; Sim, J.Y.; Collins, G.J.; Lee, J.K. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sour. Sci. Technol. 2012, 21, 43001. [Google Scholar] [CrossRef]
- Halfmann, H.; Bibinov, N.; Wunderlich, J.; Awakowicz, P. A double inductively coupled plasma for sterilization of medical devices. J. Phys. D Appl. Phys. 2007, 40, 4145–4154. [Google Scholar] [CrossRef]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Filatova, I.; Azharonok, V.; Kadyrov, M.; Belyavsky, V.; Gvodzdov, A.; Shik, A.; Antonuk, A. The effect of plasma treatment of seeds of some grain and legumes on their sowing quality and productivity. Romanian J. Phys 2011, 56, 139–143. [Google Scholar]
- Avramidis, G.; Stüwe, B.; Richard, W.; Bellmann, M.; Stephan, W.; von Tiedemann, A.; Viöl, W. Fungicidal effects of an atmospheric pressure gas discharge and degradation mechanisms. Surf. Coat. Technol. 2010, 205, 405–408. [Google Scholar] [CrossRef]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of cold atmospheric pressure plasma on the wheat seedlings vigor and on the inactivation of microorganisms on the seeds surface. Plasma Chem. Plasma Process. 2015, 36, 397–414. [Google Scholar] [CrossRef]
- Ouf, S.A.; Basher, A.H.; Mohamed, A.-A.H. Inhibitory effect of double atmospheric pressure argon cold plasma on spores and mycotoxin production of Aspergillus niger contaminating date palm fruits. J. Sci. Food Agric. 2015. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Takatori, K.; Sugita-Konishi, Y.; Kim, I.-H.; Lee, M.-H.; Han, D.-W.; Chung, K.-H.; Hyun, S.O.; Park, J.-C. Degradation of mycotoxins using microwave-induced argon plasma at atmospheric pressure. Surf. Coat. Technol. 2007, 201, 5733–5737. [Google Scholar] [CrossRef]
- Lee, H.M.; Chang, M.B. Abatement of gas-phase p-xylene via dielectric barrier discharges. Plasma Chem. Plasma Process. 2003, 23, 541–558. [Google Scholar] [CrossRef]
- Poll, H.U.; Schladitz, U.; Schreiter, S. Penetration of plasma effects into textile structures. Surf. Coat. Technol. 2001. [Google Scholar] [CrossRef]
- Kříž, P.; Olšan, P.; Havelka, Z.; Horáková, M.; Bartoš, P.; Vazdová, P.; Syamkrishna, B.; Špatenka, P. Seed Treatment and Water Purification by the Synergical Effect of Gliding Arc Plasma and Photocatalytic Film. IEEE 2014. [Google Scholar] [CrossRef]
- Kříž, P.; Petr, B.; Zbynek, H.; Jaromir, K.; Pavel, O.; Petr, S.; Miroslav, D. Influence of plasma treatment in open air on mycotoxin content and grain nutriments. Plasma Med. 2015, 5, 145–158. [Google Scholar] [CrossRef]
- Bellmann, M.; Avramidis, G.; Wascher, R.; Viöl, W. Accelerated Germination and Altered Surface Characteristics of Pisum Sativum Seeds after Plasma Treatment at Atmospheric Pressure. In Proceedings of the Conference Plasma Surface Engineering, Garmisch-Partenkirchen, Germany, 15 September 2012.
- Hopfe, V.; Sheel, D.W. Atmospheric-Pressure PECVD Coating and Plasma Chemical Etching for Continuous Processing. IEEE Trans. Plasma Sci. 2007, 35, 204–214. [Google Scholar] [CrossRef]
- Eliasson, B.; Kogelschatz, U. Nonequilibrium volume plasma chemical processing. IEEE Trans. Plasma Sci. 1991, 19, 1063–1077. [Google Scholar] [CrossRef]
- Efremov, A.M.; Kim, D.-P.; Kim, C.-I. Simple Model for Ion-Assisted Etching Using Coupled Plasma: Effect of Gas Mixing Ratio. IEEE Trans. Plasma Sci. 2004, 32, 1344–1351. [Google Scholar] [CrossRef]
- Coburn, J.W.; Winters, H.F. Ion- and electron-assisted gas-surface chemistry—An important effect in plasma etching. J. Appl. Phys. 1979, 50, 3189–3196. [Google Scholar] [CrossRef]
- Dorai, R.; Kushner, M.J. A model for plasma modification of polypropylene using atmospheric pressure discharges. J. Phys. D Appl. Phys. 2003, 36, 666–685. [Google Scholar] [CrossRef]
- Kuvaldina, E.V.; Shikova, T.G.; Smirnov, S.A.; Rybkin, V.V. Surface oxidation and degradation of polyethylene in a mixed argon-oxygen plasma. High Energy Chem. 2007, 41, 284–287. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Babayan, S.E.; Schütze, A.; Tu, V.J.; Park, J.; Henins, I.; Selwyn, G.S.; Hicks, R.F. Etching polyimide with a nonequilibrium atmospheric-pressure plasma jet. J. Vac. Sci. Technol. A 1999, 17, 2581–2585. [Google Scholar] [CrossRef]
- Park, B.J.; Lee, D.H.; Park, J.-C.; Lee, I.-S.; Lee, K.-Y.; Hyun, S.O.; Chun, M.-S.; Chung, K.-H. Sterilization using a microwave-induced argon plasma system at atmospheric pressure. Phys. Plasmas 2003, 10, 4539–4544. [Google Scholar] [CrossRef]
- Gröning, P.; Collaud, M.; Dietler, G.; Schlapbach, L. Plasma modification of polymethylmethacrylate and polyethyleneterephthalate surfaces. J. Appl. Phys. 1994, 76, 887–892. [Google Scholar] [CrossRef]
- Klarhöfer, L.; Viöl, W.; Maus-Friedrichs, W. Electron spectroscopy on plasma treated lignin and cellulose. Holzforschung 2010, 64, 313–316. [Google Scholar] [CrossRef]
- Khatibi, P.A.; Berger, G.; Wilson, J.; Brooks, W.S.; McMaster, N.; Griffey, C.A.; Hicks, K.B.; Nghiem, N.P.; Schmale, D.G. A comparison of two milling strategies to reduce the mycotoxin deoxynivalenol in barley. J. Agric. Food Chem. 2014, 62, 4204–4213. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Visentin, I.; Montis, V.; Doll, K.; Alabouvette, C.; Tamietti, G.; Karlovsky, P.; Cardinale, F. Transcription of genes in the biosynthetic pathway for fumonisin mycotoxins is epigenetically and differentially regulated in the fungal maize pathogen Fusarium verticillioides. Eukaryot. Cell 2012, 11, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.H. Variation in Fusarium graminearum and cultivar resistance to wheat scab. Plant Dis. 1996, 80, 975. [Google Scholar] [CrossRef]
- Becker, E.-M.; Herrfurth, C.; Irmisch, S.; Kollner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef] [PubMed]
- Nutz, S.; Döll, K.; Karlovsky, P. Determination of the LOQ in real-time PCR by receiver operating characteristic curve analysis: Application to qPCR assays for Fusarium verticillioides and F. proliferatum. Anal. Bioanal. Chem. 2011, 401, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, C.; Roux, S.; Brückner, S.; Wieneke, S.; Viöl, W. Low-temperature atmospheric pressure argon plasma treatment and hybrid laser-plasma ablation of barite crown and heavy flint glass. Appl. Opt. 2012, 51. [Google Scholar] [CrossRef] [PubMed]
- Ratzinger, A.; Riediger, N.; von Tiedemann, A.; Karlovsky, P. Salicylic acid and salicylic acid glucoside in xylem sap of Brassica napus infected with Verticillium longisporum. J. Plant Res. 2009, 122, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Laurentin, H.; Ratzinger, A.; Karlovsky, P. Relationship between metabolic and genomic diversity in sesame (Sesamum indicum L.). BMC Genom. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Nassar, H.; Pellerin, S.; Musiol, K.; Martinie, O.; Pellerin, N.; Cormier, J.-M. N2+/N2 ratio and temperature measurements based on the first negative N2+ and second positive N2 overlapped molecular emission spectra. J. Phys. D Appl. Phys. 2004, 37, 1904–1916. [Google Scholar] [CrossRef]
- Paris, P.; Aints, M.; Valk, F.; Plank, T.; Haljaste, A.; Kozlov, K.V.; Wagner, H.-E. Intensity ratio of spectral bands of nitrogen as a measure of electric field strength in plasmas. J. Phys. D Appl. Phys. 2005, 38, 3894–3899. [Google Scholar] [CrossRef]
- Manley, T.C. The Electric Characteristics of the Ozonator Discharge. Trans. Electrochem. Soc. 1943, 84, 83–96. [Google Scholar] [CrossRef]





| Mycotoxin | Half-Life τ [s] | Molecular Mass [Da] |
|---|---|---|
| Sterigmatocystin | 5.0 ± 0.4 | 324.3 |
| Enniatin A | 4.5 ± 0.5 | 681.9 |
| Zearalenone | 4.2 ± 0.5 | 318.4 |
| Deoxynivalenol | 4.0 ± 0.7 | 296.3 |
| T2-toxin | 3.6 ± 0.1 | 466.5 |
| Enniatin B | 3.1 ± 0.2 | 639.8 |
| AAL-toxin | 1.9 ± 0.4 | 521.6 |
| Fumonisin B1 | 1.9 ± 0.3 | 721.8 |
| Input Parameter | Value |
|---|---|
| power density | ≈4 W/cm2 |
| discharge gap | 2 mm |
| air flow | 130 sl/min |
| appl. voltage | ≈38 kV (p-p) |
| waveform | pulsed sine |
| gas temperature | Trot ≈ 330 K |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ten Bosch, L.; Pfohl, K.; Avramidis, G.; Wieneke, S.; Viöl, W.; Karlovsky, P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins 2017, 9, 97. https://doi.org/10.3390/toxins9030097
Ten Bosch L, Pfohl K, Avramidis G, Wieneke S, Viöl W, Karlovsky P. Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins. 2017; 9(3):97. https://doi.org/10.3390/toxins9030097
Chicago/Turabian StyleTen Bosch, Lars, Katharina Pfohl, Georg Avramidis, Stephan Wieneke, Wolfgang Viöl, and Petr Karlovsky. 2017. "Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species" Toxins 9, no. 3: 97. https://doi.org/10.3390/toxins9030097
APA StyleTen Bosch, L., Pfohl, K., Avramidis, G., Wieneke, S., Viöl, W., & Karlovsky, P. (2017). Plasma-Based Degradation of Mycotoxins Produced by Fusarium, Aspergillus and Alternaria Species. Toxins, 9(3), 97. https://doi.org/10.3390/toxins9030097