Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications
Abstract
:1. Introduction
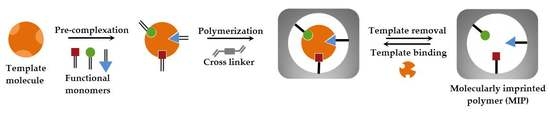
2. Fundamentals of Molecularly Imprinted Polymers (MIPs)
3. Principle of Microfluidic Systems
4. Integrations of MIPs with Microfluidic Systems
5. Latest Strategies of MIP-Based Microfluidic Systems
5.1. Polymers
5.2. Sensors
5.3. Papers
6. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Lv, Y.Q.; Tan, T.W.; Svec, F. Molecular imprinting of proteins in polymers attached to the surface of nanomaterials for selective recognition of biomacromolecules. Biotechnol. Adv. 2013, 31, 1172–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fan, L.; Dai, Y.; Kan, X. Recognition and determination of bovine hemoglobin using a gold electrode modified with gold nanoparticles and molecularly imprinted self-polymerized dopamine. Microchim. Acta 2015, 182, 2477–2483. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Use of polymers with enzyme analogous structures for the resolution of racemates. Angew. Chem. Int. Ed. 1972, 11, 341. [Google Scholar]
- Saylan, Y.; Tamahkar, E.; Denizli, A. Recognition of lysozyme using surface imprinted bacterial cellulose nanofibers. J. Biomat. Sci. Polym. Ed. 2017, 28, 1950–1965. [Google Scholar] [CrossRef] [PubMed]
- Safran, V.; Göktürk, I.; Derazshamshir, A.; Yılmaz, F.; Sağlam, N.; Denizli, A. Rapid sensing of Cu2+ in water and biological samples by sensitive molecularly imprinted based plasmonic biosensor. Microchem. J. 2019, 148, 141–150. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Yavuz, H.; Denizli, A. Controlled release of mitomycin C from PHEMAH–Cu(II) cryogel membranes. J. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Cabaleiro-Lago, C.; Quinlan-Pluck, F.; Lynch, I.; Lindman, S.; Minogue, A.M.; Thulin, E.; Walsh, D.M.; Dawson, K.A.; Linse, S. Inhibition of amyloid β protein fibrillation by polymeric nanoparticles. J. Am. Chem. Soc. 2008, 130, 15437–15443. [Google Scholar] [CrossRef] [PubMed]
- Çetin, K.; Denizli, A. 5-Fluorouracil delivery from metal-ion mediated molecularly imprinted cryogel discs. Colloids Surf. B Biointerfaces 2015, 126, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.C.; Lemcoff, N.G. Synthetic hosts via molecular imprinting-are universal synthetic antibodies realistically possible? Chem. Commun. 2004, 1, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Golabi, M.; Kuralay, F.; Jager, E.W.H.; Beni, V.; Turner, A.P.F. Electrochemical bacterial detection using poly(3-aminophenylboronic acid)-based imprinted polymer. Biosens. Bioelectron. 2017, 93, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Saylan, Y.; Yılmaz, F.; Özgür, E.; Derazshamshir, A.; Yavuz, H.; Denizli, A. Molecularly imprinting of macromolecules for sensors applications. Sensors 2017, 17, 898. [Google Scholar] [CrossRef] [PubMed]
- Mosbach, K. The promise of molecular imprinting. Sci. Am. 2006, 295, 86. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Manz, A.; Harrison, D.J.; Verpoorte, E.M.J.; Fettinger, J.C.; Paulus, A.; Lüdi, H.; Widmer, H.M. Planar chips technology for miniaturization and integration of separation techniques into monitoring systems: Capillary electrophoresis on a chip. J. Chromatog. A 1992, 593, 253–258. [Google Scholar] [CrossRef]
- Chin, C.D.; Linder, V.; Sia, S.K. Commercialization of microfluidic point-of-care diagnostic devices. Lab Chip 2012, 12, 2118–2134. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.Y.; Lin, R.; Hung, L.H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Sia, S.K.; Kricka, L.J. Microfluidics and point-of-care testing. Lab Chip 2008, 8, 1982–1983. [Google Scholar] [CrossRef] [PubMed]
- Lifson, M.A.; Ozen, M.O.; Inci, F.; Wang, S.; Inan, H.; Baday, M.; Henrich, T.J.; Demirci, U. Advances in biosensing strategies for HIV-1 detection, diagnosis, and therapeutic monitoring. Adv. Drug Deliv. Rev. 2016, 103, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Vera, F.; Zenuto, R.R.; Antenucci, C.D.; Busso, J.M.; Marín, R.H. Validation of a radioimmunoassay for measuring testosterone concentrations in plasma samples of the subterranean rodent Ctenomys talarum: Outstandingly elevated levels in the wild and the effect of captivity. J. Exp. Zool. 2011, 315, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, R.L.; Griffin, T.L.; Herold, D.A. Analysis of testosterone in serum using mass spectrometry. Methods Mol. Biol. 2010, 603, 489–500. [Google Scholar] [PubMed]
- Wang, Y.; Gay, G.D.; Botelho, J.C.; Caudill, S.P.; Vesper, H.W. Total testosterone quantitative measurement in serum by LC-MS/MS. Clin. Chim. Acta 2014, 436, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Ye, L. Synthetic strategies in molecular imprinting. In Advances in Biochemical Engineering/Biotechnology; Springer: Berlin, Germany, 2015. [Google Scholar]
- Saylan, Y.; Denizli, A. Molecular fingerprints of hemoglobin on a nanofilm chip. Sensors 2018, 18, 3016. [Google Scholar] [CrossRef] [PubMed]
- Gast, M.; Sobek, H.; Mizaikoff, B. Advances in imprinting strategies for selective virus recognition a review. Trend Anal. Chem. 2019, 114, 218–232. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly imprinted polymer based sensors for medical applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhang, Z.; Li, J.; Ma, X.; Chen, L.; Yang, X. Molecular imprinting technology for microorganism analysis. Trend Anal. Chem. 2018, 106, 190–201. [Google Scholar] [CrossRef]
- Yan, H.; Row, K.R. Characteristic and synthetic approach of molecularly imprinted polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Uzun, L.; Turner, A.P.F. Molecularly-imprinted polymer sensors: Realizing their potential. Biosens. Bioelectron. 2016, 76, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R.; Qian, J.; Dickert, F.L. Immunosensing with artificial antibodies in organic solvents or complex matrices. Sens. Actuators B 2012, 173, 585–590. [Google Scholar] [CrossRef]
- Erdem, Ö.; Saylan, Y.; Cihangir, N.; Denizli, A. Molecularly imprinted nanoparticles based plasmonic sensors for real-time Enterococcus faecalis detection. Biosens. Bioelectron. 2019, 126, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Middeleer, G.D.; Dubruel, P.; Saeger, S.D. Characterization of MIP and MIP functionalized surfaces: Current state-of-the-art. Trend Anal. Chem. 2016, 76, 71–85. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Rocha Santos, T.A.P. Recent developments in recognition elements for chemical sensors and biosensors. Trend Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Choi, J.R.; Yong, K.W.; Choi, J.Y.; Cowie, A.C. Progress in molecularly imprinted polymers for biomedical applications. Comb. Chem. High Throughput Screen. 2019, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Masliyah, J.H.; Bhattacharjee, S. Electrokinetic and Colloid Transport Phenomena; John Wiley and Sons: Hoboken, NY, USA, 2006. [Google Scholar]
- Nobes, D.S.; Abdolrazaghi, M.; Mitra, S.K. Microfluidics and Nanofluidics Handbook, Fabrication, Implementation, and Applications, Image-Based Photonic Techniques for Microfluidics; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Vasdekis, A.E.; Laporte, G.P.J. Enhancing single molecule imaging in optofluidics and microfluidics. Int. J. Mol. Sci. 2011, 12, 5135–5156. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms: Requirements, Characteristics and Applications. Microfluidics Based Microsystems; Springer: Berlin, Germany, 2010. [Google Scholar]
- Gervais, L.; Rooij, N.D.; Delamarche, E. Microfluidic chips for point-of-care immunodiagnostics. Adv. Mater. 2011, 23, H151–H176. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Schirhagl, R.; Ren, K.N.; Zare, R.N. Surface-Imprinted Polymers in Microfluidic Devices; Springer: Berlin, Germany, 2012. [Google Scholar]
- Krupadam, R.J.; Korde, B.A.; Ashokkumar, M.; Kolev, S.D. Novel molecularly imprinted polymeric microspheres for preconcentration and preservation of polycyclic aromatic hydrocarbons from environmental samples. Anal. Bioanal. Chem. 2014, 406, 5313–5321. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Lin, C.C.; Hong, C.L.; Chang, P.H. Enhanced anesthetic propofol biochips by modifying molecularly imprinted nanocavities of biosensors. Biomed. Microdevices 2012, 14, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Rico-Yuste, A.; Carrasco, S. Molecularly imprinted polymer-based hybrid materials for the development of optical sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regan, B.; Boyle, F.; O’Kennedy, R.; Collins, D. Evaluation of molecularly imprinted polymers for point-of-care testing for cardiovascular disease. Sensors 2019, 19, 3485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Horikawa, S.; Venkatesh, S.; Saha, J.; Hong, J.W.; Byrne, M.E. Zero-order therapeutic release from imprinted hydrogel contact lenses within in vitro physiological ocular tear flow. J. Control. Release 2007, 124, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Shiraki, Y.; Tsuruta, K.; Morimoto, J.; Ohba, J.; Kawamura, A.; Yoshida, R.; Kawano, R.; Uragami, T.; Miyata, T. Preparation of molecule-responsive microsized hydrogels via photopolymerization for smart microchannel microvalves. Macromol. Rapid Commun. 2015, 36, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, K.; Takano, E.; Kitayama, Y.; Takeuchi, T. Synthesis of monodispersed submillimeter-sized molecularly imprinted particles selective for human serum albumin using inverse suspension polymerization in water-in-oil emulsion prepared using microfluidics. Langmuir 2015, 31, 4981–4987. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zare, R.N. Chemical recognition in cell-imprinted polymers. ACS Nano 2012, 6, 4314–4318. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Chen, C.P.; Horng, C.J.; Chen, S.Y. Point-of-care protein sensing platform based on immuno-like membrane with molecularly-aligned nanocavities. Biosens. Bioelectron. 2013, 50, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Kellens, E.; Bové, H.; Vandenryt, T.; Lambrichts, J.; Dekens, J.; Drijkoningen, S.; D’Haen, J.; De Ceuninck, W.; Thoelen, R.; Junkers, T.; et al. Micro-patterned molecularly imprinted polymer structures on functionalized diamond-coated substrates for testosterone detection. Biosens. Bioelectron. 2018, 118, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Thaler, M.; Luppa, P.B. Highly sensitive immunodiagnostics at the point of care employing alternative recognition elements and smartphones: Hype, trend, or revolution? Anal. Bioanal. Chem. 2019, 2019, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Harz, S.; Schimmelpfennig, M.; Bui, B.T.S.; Marchyk, N.; Haupt, K.; Feller, K.H. Fluorescence optical spectrally resolved sensor based on molecularly imprinted polymers and microfluidics. Eng. Life Sci. 2011, 11, 559–565. [Google Scholar] [CrossRef]
- Sharma, P.S.; Iskierko, Z.; Noworyta, K.; Cieplak, M.; Borowicz, P.; Lisowski, W.; D’Souza, F.; Kutner, W. Synthesis and application of a “plastic antibody” in electrochemical microfluidic platform for oxytocin determination. Biosens. Bioelectron. 2018, 100, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Jiang, M.; Tian, L.; Sun, S.; Zhao, N.; Zhao, F.; Li, Y. Electrochemical microfluidic chip based on molecular imprinting technique applied for therapeutic drug monitoring. Biosens. Bioelectron. 2017, 91, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Yeh, W.M.; Ho, K.C.; Lee, G.B. A microfluidic system utilizing molecularly imprinted polymer films for amperometric detection of morphine. Sens. Actuator B 2007, 121, 576–582. [Google Scholar] [CrossRef]
- Hong, C.C.; Lin, C.C.; Hong, C.L.; Lin, Z.X.; Chung, M.H.; Hsieh, P.W. Handheld analyzer with on-chip molecularly-imprinted biosensors for electrical detection of propofol in plasma samples. Biosens. Bioelectron. 2016, 86, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Chang, P.H.; Lin, C.C.; Hong, C.L. A disposable microfluidic biochip with on-chip molecularly imprinted biosensors for optical detection of anesthetic propofol. Biosens. Bioelectron. 2010, 25, 2058–2064. [Google Scholar] [CrossRef] [PubMed]
- Dejous, C.; Hallil, H.; Raimbault, V.; Lachaud, J.; Plano, B.; Delépée, R.; Favetta, P.; Agrofoglio, L.; Rebière, D. Love acoustic wave-based devices and molecularly-imprinted polymers as versatile sensors for electronic nose or tongue for cancer monitoring. Sensors 2016, 16, 915. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.M.; Martinez, A.W.; Gong, J.; Mace, C.R.; Phillips, S.T.; Carrilho, E.; Mirica, K.A.; Whitesides, G.M. Paper-based ELISA. Angew. Chem. Int. Ed. 2010, 49, 4771–4774. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Crooks, R.M. Three-dimensional paper microfluidic devices assembled using the principles of origami. J. Am. Chem. Soc. 2011, 133, 17564–17566. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wei, X.; Jia, S.; Zhang, R.; Li, J.; Zhu, Z.; Zhang, H.; Ma, Y.; Lin, Z.; Yang, C.J. Integration of target responsive hydrogel with cascaded enzymatic reactions and microfluidic paper-based analytic devices (PADs) for point-of-care testing (POCT). Biosens. Bioelectron. 2016, 77, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, Y.; Zhang, L.; Ge, S.; Yu, J. A novel microfluidic paper-based colorimetric sensor based on molecularly imprinted polymer membranes for highly selective and sensitive detection of bisphenol A. Sens. Actuators B 2017, 243, 130–136. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, Y.; Zhang, L.; Xu, C.; Yu, J. Highly sensitive microfluidic paper-based photoelectrochemical sensing platform based on reversible photo-oxidation products and morphology preferable multi-plate ZnO nanoflowers. Biosens. Bioelectron. 2018, 110, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, Z.; Qi, J.; Zhou, N.; Qin, S.; Choo, J.; Chen, L. Quantum dot-based molecularly imprinted polymers on three-dimensional origami paper microfluidic chip for fluorescence detection of phycocyanin. ACS Sens. 2017, 2, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Li, B.; Wang, X.; Zhang, Z.; Wang, Z.; Han, J.; Chen, L. Three-dimensional paper-based microfluidic chip device for multiplexed fluorescence detection of Cu2+ and Hg2+ ions based on ion imprinting technology. Sens. Actuators B 2017, 251, 224–233. [Google Scholar] [CrossRef]
- Ge, L.; Wang, S.; Yu, J.; Li, N.; Ge, S.; Yan, M. Molecularly imprinted polymer grafted porous Au-paper electrode for a microfluidic electro-analytical origami device. Adv. Funct. Mater. 2013, 23, 3115–3123. [Google Scholar] [CrossRef]










| Material | Target | Advantages | Dynamic Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| Hydrogel | Ketotifen fumarate | A potential determination of physiological release rates, matching local conditions to characterize drug delivery devices | 4.04 × 10−9 to 5.57 × 10−10 cm2/s | – | [45] |
| Hydrogel | Bisphenol A | An ultra-fast shrinkage in response, adjusted flow rate by the shrinking of the hydrogels | 120 μg/mL | – | [46] |
| Microgel | Human serum albumin | A high affinity, selectivity and stability | 5 μM | – | [47] |
| Film | Staphylococcus epidermidis, Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae | One sorting cycle to capture, release a pure bacterial strain, the dominant role of chemical recognition | 109 cells/mL | – | [48] |
| Membrane | C-reactive protein | A specific and cost-effective approach, catch specific proteins in complex | 0–200 μg/mL | – | [49] |
| Microstructure | Testosterone | A low-cost, simple, robust, efficient, less time consuming | 0.5–500 nM | 0.5 nM | [50] |
| Fluorescence | Dansyl-L-phenylalanine | A high sensitivity and selectivity | 1–100 μM | 0.5 μM | [52] |
| Electrochemical | Oxytocin nonapeptide | A high sensitivity and selectivity | 0.06–1 mM | 60 μM | [53] |
| Electrochemical | Warfarin sodium | An accurate, reliable, interference-free, simple, low-cost | 2 × 10−11 to 4 × 10−9 M | 8 × 10−12 M | [54] |
| Electrochemical | Morphine | A precise and continuous measurement, compact in size, consumes fewer samples | 0.01–0.2 mM | 0.3 μM | [55] |
| Electrochemical | Propofol | A compact size, high selectivity, low cost, rapid response, single-step detection | 0.1–30 μg/mL | 0.1 μg/mL | [56] |
| Optical | Propofol | A disposable, high selectivity, low cost, rapid response, single-step detection | 0.25–10 ppm | 0.25 ppm | [57] |
| Film | Adenosine-51-monophosphate | A detection in real-time, low concentrations of nucleoside analogues, good stability | 5–600 ppm | 5 ppm | [58] |
| Magnetic nanoparticle | Bisphenol A | A highly reproducible response, good selectivity, excellent regeneration | 10–1000 nM | 6.18 nM | [63] |
| Nanoflowers | L-glutamic acid and L-cysteine | A selective, accurate, rapid, inexpensive, on-site monitoring | 20 pM to 1000 nM and 50 pM to 800 nM | 9.6 pM and 24 pM | [64] |
| Quantum dot | Phycocyanin | A robust, facile route to detection, portability, disposability, low cost, user-friendly protocol | 10−50 mg/L | 2 mg/L | [65] |
| Quantum dot | Cu2+ and Hg2+ ions | A novel, simple, convenient analysis, cost-effective, portable | 0.11 to 58.0 µg/L (Cu2+) and 0.26–34.0 µg/L (Hg2+) | 0.035 µg/L (Cu2+) and 0.056 µg/L (Hg2+) | [66] |
| Gold nanoparticle | D-glutamic acid | A high-throughput, sensitive, specific, multiplex assay | 1.2−125.0 nM | 0.2 nM | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saylan, Y.; Denizli, A. Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications. Micromachines 2019, 10, 766. https://doi.org/10.3390/mi10110766
Saylan Y, Denizli A. Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications. Micromachines. 2019; 10(11):766. https://doi.org/10.3390/mi10110766
Chicago/Turabian StyleSaylan, Yeşeren, and Adil Denizli. 2019. "Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications" Micromachines 10, no. 11: 766. https://doi.org/10.3390/mi10110766
APA StyleSaylan, Y., & Denizli, A. (2019). Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications. Micromachines, 10(11), 766. https://doi.org/10.3390/mi10110766