Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Reference Method
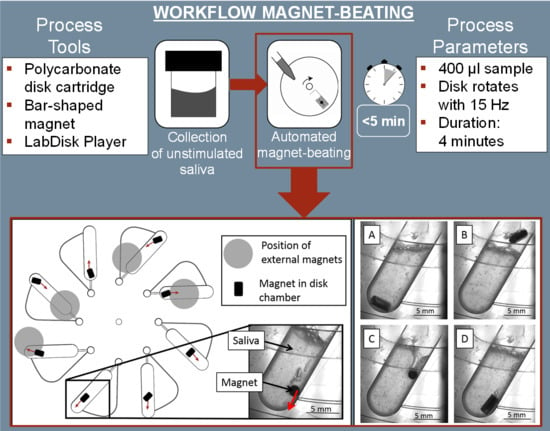
2.3. Automated Centrifugal Microfluidic System
2.4. Fluidic and Biochemical Characterization of Samples
2.5. Design of Experiments (DoE)
3. Results and Discussion
3.1. Magnet-Beating Operating Principle
3.2. Design of Experiments Analysis for Magnet-Beating Optimization
3.3. Impact of Magnet-Beating on the Fluidic Behavior of Whole Saliva
3.4. Impact of Magnet-Beating on Protein Biochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Details on the Design of Experiments Analysis

References
- Humphrey, S.P.; Williamson, R.T. A review of saliva: normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Slowey, P.D. Saliva Collection Devices and Diagnostic Platforms. In Advances in Salivary Diagnostics; Streckfus, C.F., Ed.; Springer Berlin Heidelberg: Berlin, Germany, 2015; pp. 33–61. [Google Scholar]
- Malamud, D. Saliva as a diagnostic fluid. Dent. Clin. North Am. 2011, 55, 159–178. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Nayak, P.A.; Rana, S. Point of Care- A Novel Approach to Periodontal Diagnosis-A Review. J. Clin. Diagn. Res. 2017, 11, ZE01–ZE06. [Google Scholar] [PubMed]
- Bostanci, N.; Selevsek, N.; Wolski, W.; Grossmann, J.; Bao, K.; Wahlander, A.; Trachsel, C.; Schlapbach, R.; Öztürk, V.Ö.; Afacan, B.; et al. Targeted Proteomics Guided by Label-free Quantitative Proteome Analysis in Saliva Reveal Transition Signatures from Health to Periodontal Disease. Mol. Cell. Proteomics 2018, 17, 1392–1409. [Google Scholar] [CrossRef] [PubMed]
- Silbereisen, A.; Hallak, A.K.; Nascimento, G.G.; Sorsa, T.; Belibasakis, G.N.; Lopez, R.; Bostanci, N. Regulation of PGLYRP1 and TREM-1 during Progression and Resolution of Gingival Inflammation. JDR Clin. Trans. Res. 2019, 4, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Oztürk, V.Ö.; Emingil, G.; Belibasakis, G.N. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in periodontitis. J. Dent. Res. 2013, 92, 161–165. [Google Scholar] [CrossRef]
- Nylund, K.M.; Ruokonen, H.; Sorsa, T.; Heikkinen, A.M.; Meurman, J.H.; Ortiz, F.; Tervahartiala, T.; Furuholm, J.; Bostanci, N. Association of the salivary triggering receptor expressed on myeloid cells/its ligand peptidoglycan recognition protein 1 axis with oral inflammation in kidney disease. J. Periodontol. 2018, 89, 117–129. [Google Scholar] [CrossRef]
- Grassl, N.; Kulak, N.A.; Pichler, G.; Geyer, P.E.; Jung, J.; Schubert, S.; Sinitcyn, P.; Cox, J.; Mann, M. Ultra-deep and quantitative saliva proteome reveals dynamics of the oral microbiome. Genome Med. 2016, 8, 44. [Google Scholar] [CrossRef]
- Golatowski, C.; Salazar, M.G.; Dhople, V.M.; Hammer, E.; Kocher, T.; Jehmlich, N.; Völker, U. Comparative evaluation of saliva collection methods for proteome analysis. Clin. Chim. Acta 2013, 419, 42–46. [Google Scholar]
- Dawes, C. Considerations in the development of diagnostic tests on saliva. Ann. N. Y. Acad. Sci. 1993, 694, 265–269. [Google Scholar] [CrossRef]
- Herr, A.E.; Hatch, A.V.; Throckmorton, D.J.; Tran, H.M.; Brennan, J.S.; Giannobile, W.V.; Singh, A.K. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc. Natl. Acad. Sci. USA 2007, 104, 5268–5273. [Google Scholar] [CrossRef]
- Dawes, C.; Wong, D.T.W. Role of Saliva and Salivary Diagnostics in the Advancement of Oral Health. J. Dent. Res. 2019, 98, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, W.H. The rheology of saliva. J. Dent. Res. 1987, 66, 660–666. [Google Scholar] [CrossRef] [PubMed]
- van der Reijden, W.A.; Veerman, E.C.; Amerongen, A.V. Shear rate dependent viscoelastic behavior of human glandular salivas. Biorheology 1993, 30, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Helton, K.L.; Yager, P. Interfacial instabilities affect microfluidic extraction of small molecules from non-Newtonian fluids. Lab Chip 2007, 7, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Amado, F.M.L.; Vitorino, R.M.P.; Domingues, P.M.D.N.; Lobo, M.J.C.; Duarte, J.A.R. Analysis of the human saliva proteome. Expert Rev. Proteomics 2005, 2, 521–539. [Google Scholar] [CrossRef]
- Gug, I.T.; Tertis, M.; Hosu, O.; Cristea, C. Salivary biomarkers detection: Analytical and immunological methods overview. TrAC-Trends Anal. Chem. 2019, 113, 301–316. [Google Scholar] [CrossRef]
- Rantonen, P.J.; Meurman, J.H. Viscosity of whole saliva. Acta Odontol. Scand. 1998, 56, 210–214. [Google Scholar] [CrossRef]
- Schramm, W.; Annesley, T.M.; Siegel, G.J.; Sackellares, J.C.; Smith, R.H. Measurement of phenytoin and carbamazepine in an ultrafiltrate of saliva. Ther. Drug Monit. 1991, 13, 452–460. [Google Scholar] [CrossRef]
- Helton, K.L.; Nelson, K.E.; Fu, E.; Yager, P. Conditioning saliva for use in a microfluidic biosensor. Lab Chip 2008, 8, 1847–1851. [Google Scholar] [CrossRef]
- Spielmann, N.; Wong, D.T. Saliva: diagnostics and therapeutic perspectives. Oral Dis. 2011, 17, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Schneyer, L.H. Coagulation of salivary mucoid by freezing and thawing of saliva. Proc. Soc. Exp. Biol. Med. 1956, 91, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Al-Tarawneh, S.K.; Border, M.B.; Dibble, C.F.; Bencharit, S. Defining salivary biomarkers using mass spectrometry-based proteomics: a systematic review. OMICS 2011, 15, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.; Koh, D.; Fu, Q.; Chia, S.-E. Effects of storage time on stability of salivary immunoglobulin A and lysozyme. Clin. Chim. Acta 2003, 338, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.W.; Mauk, M.G.; Liu, C.; Qiu, X.; Thompson, J.A.; Chen, D.; Malamud, D.; Abrams, W.R.; Bau, H.H. Point-of-care oral-based diagnostics. Oral Dis. 2011, 17, 745–752. [Google Scholar] [CrossRef]
- Mitsakakis, K.; Stumpf, F.; Strohmeier, O.; Klein, V.; Mark, D.; von Stetten, F.; Peham, J.R.; Herz, C.; Tawakoli, P.N.; Wegehaupt, F.; et al. Chair/bedside diagnosis of oral and respiratory tract infections, and identification of antibiotic resistances for personalised monitoring and treatment. Stud. Health Technol. Inform. 2016, 224, 61–66. [Google Scholar]
- Wei, F.; Patel, P.; Liao, W.; Chaudhry, K.; Zhang, L.; Arellano-Garcia, M.; Hu, S.; Elashoff, D.; Zhou, H.; Shukla, S.; et al. Electrochemical sensor for multiplex biomarkers detection. Clin. Cancer Res. 2009, 15, 4446–4452. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, C.-C.; Peng, Y.-S.; Wu, C.-W.; Chang, Y.-T.; Chang, K.-P. Detection of anti-p53 autoantibodies in saliva using microfluidic chips for the rapid screening of oral cancer. RSC Adv. 2018, 8, 15513–15521. [Google Scholar] [CrossRef]
- Nie, S.; Henley, W.H.; Miller, S.E.; Zhang, H.; Mayer, K.M.; Dennis, P.J.; Oblath, E.A.; Alarie, J.P.; Wu, Y.; Oppenheim, F.G.; et al. An automated integrated platform for rapid and sensitive multiplexed protein profiling using human saliva samples. Lab Chip 2014, 14, 1087–1098. [Google Scholar] [CrossRef]
- Christodoulides, N.; Floriano, P.N.; Miller, C.S.; Ebersole, J.L.; Mohanty, S.; Dharshan, P.; Griffin, M.; Lennart, A.; Ballard, K.L.M.; King, C.P.; et al. Lab-on-a-chip methods for point-of-care measurements of salivary biomarkers of periodontitis. Ann. N. Y. Acad. Sci. 2007, 1098, 411–428. [Google Scholar] [CrossRef]
- Nwhator, S.O.; Ayanbadejo, P.O.; Umeizudike, K.A.; Opeodu, O.I.; Agbelusi, G.A.; Olamijulo, J.A.; Arowojolu, M.O.; Sorsa, T.; Babajide, B.S.; Opedun, D.O. Clinical correlates of a lateral-flow immunoassay oral risk indicator. J. Periodontol. 2014, 85, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kido, H.; Micic, M.; Smith, D.; Zoval, J.; Norton, J.; Madou, M. A novel, compact disk-like centrifugal microfluidics system for cell lysis and sample homogenization. Colloids Surf. B Biointerfaces 2007, 58, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, J.; Gorkin, R.; Bastien, M.; Stewart, G.; Peytavi, R.; Kido, H.; Bergeron, M.; Madou, M. Validation of a centrifugal microfluidic sample lysis and homogenization platform for nucleic acid extraction with clinical samples. Lab Chip 2010, 10, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Focke, M.; Stumpf, F.; Faltin, B.; Reith, P.; Bamarni, D.; Wadle, S.; Müller, C.; Reinecke, H.; Schrenzel, J.; Francois, P.; et al. Microstructuring of polymer films for sensitive genotyping by real-time PCR on a centrifugal microfluidic platform. Lab Chip 2010, 10, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Hin, S.; Paust, N.; Rombach, M.; Lueddecke, J.; Specht, M.; Zengerle, R.; Mitsakakis, K. Minimizing Ethanol Carry-Over in Centrifugal Microfluidic Nucleic Acid Extraction by Advanced Bead Handling and Management of Diffusive Mass Transfer. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII, Berlin, Germany, 23–27 June 2019; pp. 130–133. [Google Scholar]
- Cui, F.; Rhee, M.; Singh, A.; Tripathi, A. Microfluidic Sample Preparation for Medical Diagnostics. Annu. Rev. Biomed. Eng. 2015, 17, 267–286. [Google Scholar] [CrossRef]
- Kashket, S.; Ciociolo, J.M. Gel electrophoresis salivary mucins. Electrophoresis 1981, 2, 55–59. [Google Scholar] [CrossRef]
- Offner, G.D.; Troxler, R.F. Heterogeneity of high-molecular-weight human salivary mucins. Adv. Dent. Res. 2000, 14, 69–75. [Google Scholar] [CrossRef]
- Takehara, S.; Yanagishita, M.; Podyma-Inoue, K.A.; Kawaguchi, Y. Degradation of MUC7 and MUC5B in human saliva. PLoS ONE 2013, 8, e69059. [Google Scholar] [CrossRef] [Green Version]
- Bruno, L.S.; Li, X.; Wang, L.; Soares, R.V.; Siqueira, C.C.; Oppenheim, F.G.; Troxler, R.F.; Offner, G.D. Two-hybrid analysis of human salivary mucin MUC7 interactions. Biochim. Biophys. Acta 2005, 1746, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Iontcheva, I.; Oppenheim, F.G.; Troxler, R.F. Human salivary mucin MG1 selectively forms heterotypic complexes with amylase, proline-rich proteins, statherin, and histatins. J. Dent. Res. 1997, 76, 734–743. [Google Scholar] [CrossRef]
- Papale, M.; Pedicillo, M.C.; Di Paolo, S.; Thatcher, B.J.; Lo Muzio, L.; Bufo, P.; Rocchetti, M.T.; Centra, M.; Ranieri, E.; Gesualdo, L. Saliva analysis by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (SELDI-TOF/MS): from sample collection to data analysis. Clin. Chem. Lab. Med. 2008, 46, 89–99. [Google Scholar] [CrossRef] [PubMed]




| Treatment | Shear rate [1/s] | Mean viscosity [mPa s] | Standard deviation [mPa s] |
|---|---|---|---|
| None | 50 | 10.4 | 4.1 |
| 3000 | 1.9 | 0.3 | |
| Reference method | 50 | 2.7 | 0.8 |
| 3000 | 1.4 | 0.1 | |
| Magnet-beating | 50 | 2.3 | 0.9 |
| 3000 | 1.4 | 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johannsen, B.; Müller, L.; Baumgartner, D.; Karkossa, L.; Früh, S.M.; Bostanci, N.; Karpíšek, M.; Zengerle, R.; Paust, N.; Mitsakakis, K. Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis. Micromachines 2019, 10, 833. https://doi.org/10.3390/mi10120833
Johannsen B, Müller L, Baumgartner D, Karkossa L, Früh SM, Bostanci N, Karpíšek M, Zengerle R, Paust N, Mitsakakis K. Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis. Micromachines. 2019; 10(12):833. https://doi.org/10.3390/mi10120833
Chicago/Turabian StyleJohannsen, Benita, Lara Müller, Desirée Baumgartner, Lena Karkossa, Susanna M. Früh, Nagihan Bostanci, Michal Karpíšek, Roland Zengerle, Nils Paust, and Konstantinos Mitsakakis. 2019. "Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis" Micromachines 10, no. 12: 833. https://doi.org/10.3390/mi10120833
APA StyleJohannsen, B., Müller, L., Baumgartner, D., Karkossa, L., Früh, S. M., Bostanci, N., Karpíšek, M., Zengerle, R., Paust, N., & Mitsakakis, K. (2019). Automated Pre-Analytic Processing of Whole Saliva Using Magnet-Beating for Point-of-Care Protein Biomarker Analysis. Micromachines, 10(12), 833. https://doi.org/10.3390/mi10120833