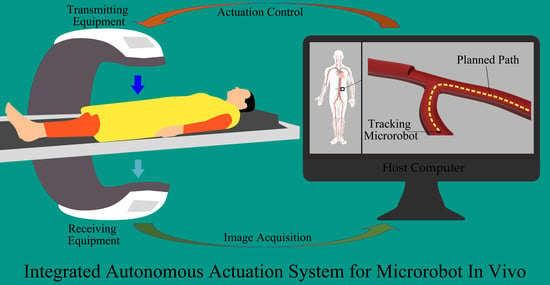
A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo
Abstract
:1. Introduction
2. Microrobots’s Actuation
2.1. Magnetic Field Actuation
2.2. Light Field Actuation
2.3. Acoustic Field Actuation
2.4. Electric Field Actuation
2.5. Hybrid Actuation
3. Microrobot Imaging
3.1. Magnetic Field-Based Imaging
3.2. Optical Imaging
3.3. Ultrasound Imaging
3.4. Ionizing Radiation Imaging
3.5. Photoacoustic Imaging
3.6. Multimodal Imaging
4. Actuation and Imaging Integration
4.1. Shared Transmission Equipment
4.2. Independent Transmission Equipment
5. Microrobot’s Medical Imaging-Based Autonomous Actuation Control
5.1. Path Planning
5.2. Imaging-Guided Autonomous Actuation
6. Conclusions and Outlook
6.1. Actuation
6.2. Imaging
6.3. Actuation and Imaging Integration
6.4. Medical Imaging-Based Autonomous Navigation Control
6.5. System Integration for Autonomous Actuation In Vivo
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in Micro-/Nanorobotics: Materials Development, Actuation, Localization, and System Integration for Biomedical Applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef]
- Lim, M.; Goos, J.A.C.M.; Qiao, R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Pharmacol. 2018, 9, 831. [Google Scholar] [CrossRef] [Green Version]
- Randhawa, J.S.; Kadam, S.; Yamanaka, S.; Selaru, F.M.; Shin, E.J.; Kalloo, A.N.; Gracias, D.H. Biopsy with Thermally-Responsive Untethered Microtools. Adv. Mater. 2013, 25, 514–519. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Go, G.; Lee, C.; Lim, K.S.; Sim, D.S.; Jeong, M.H.; Ko, S.Y.; Park, J.O.; Park, S. Penetration of an artificial arterial thromboembolism in a live animal using an intravascular therapeutic microrobot system. Med. Eng. Phys. 2016, 38, 403–410. [Google Scholar] [CrossRef]
- Soh, H.T. Switch-based biosensors: A new approach towards real-time, in vivo molecular detection. Trends Biotechnol. 2011, 29, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Troll, J.; Jeong, H.H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Huang, Q.; Shi, Q.; Yue, T.; Chen, S.; Nakajima, M.; Takeuchi, M.; Fukuda, T. Automated Assembly of Vascular-Like Microtube With Repetitive Single-Step Contact Manipulation. IEEE Trans. Biomed. Eng. 2015, 62, 2620–2628. [Google Scholar] [CrossRef]
- Du, X.; Cui, H.; Xu, T.; Huang, C.; Wang, Y.; Zhao, Q.; Xu, Y.; Wu, X. Reconfiguration, Camouflage, and Color-Shifting for Bioinspired Adaptive Hydrogel-Based Millirobots. Adv. Funct. Mater. 2020, 30, 1909202. [Google Scholar] [CrossRef]
- Yasa, I.C.; Kilic, U.; Hu, W.; Sitti, M. Translational prospects of untethered medical microrobots. Prog. Biomed. Eng. 2019, 1, 012002. [Google Scholar] [CrossRef]
- Peng, F.; Tu, Y.; Wilson, D.A. Micro- and nano-motors for biomedical applications. J. Mater. Chem. B 2014, 2, 2395–2408. [Google Scholar] [CrossRef]
- Grammatikopoulou, M.; Yang, G.Z. Laser-Printing and 3D Optical-Control of Untethered Microrobots. Adv. Opt. Mater. 2017, 5, 1700031. [Google Scholar] [CrossRef]
- Mathieu, J.B.; Beaudoin, G.; Martel, S. In Vivo MR-Tracking Based on Magnetic Signature Selective Excitation. IEEE Trans. Med Imaging 2008, 27, 28–35. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Wang, L.; Shen, Y. Micro-rocket robot with all-optic actuating and tracking in blood. Light. Sci. Appl. 2020, 9, 84. [Google Scholar] [CrossRef]
- Wan, Y.; Song, S. RectMag3D: A Magnetic Actuation System for Steering Milli/Microrobots Based on Rectangular Electromagnetic Coils. Appl. Sci. 2020, 10, 2677. [Google Scholar] [CrossRef] [Green Version]
- Abbott, J.J.; Dong, L.; Peyer, K.E.; Kratochvil, B.E.; Zhang, H.; Bergeles, C.; Nelson, B.J. Characterizing the Swimming Properties of Artificial Bacterial Flagella. Nano Lett. 2009, 9, 3663–3667. [Google Scholar] [CrossRef]
- Wang, H.; Dong, L.; Shi, Q.; Li, J.; Sun, T.; Huang, Q.; Fukuda, T. Ionic shape-morphing microrobotic end-effectors for environmentally adaptive targeting, releasing, and sampling. Nat. Commun. 2021, 12, 411. [Google Scholar] [CrossRef]
- Xu, T.; Hwang, G.; Andreff, N.; Régnier, S. Planar Path Following of 3-D Steering Scaled-Up Helical Microswimmers. IEEE Trans. Robot. 2015, 31, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liu, J.; Huang, C.; Su, M.; Xu, T. 3-D Path Following of Helical Microswimmers With an Adaptive Orientation Compensation Model. IEEE Trans. Autom. Sci. Eng. 2020, 17, 823–832. [Google Scholar] [CrossRef]
- Xu, T.; Guan, Y.; Liu, J.; Wu, X. Image-Based Visual Servoing of Helical Microswimmers for Planar Path Following. IEEE Trans. Autom. Sci. Eng. 2020, 17, 325–333. [Google Scholar] [CrossRef]
- Pane, S.; Iacovacci, V.; Koukourakis, N.; Czarske, J.; Menciassi, A.; Medina-Sánchez, M.; Schmidt, O.G. Medical Imaging of Microrobots: Toward In Vivo Applications. ACS Nano 2020, 14, 10865–10893. [Google Scholar] [CrossRef]
- Arai, F. Self-Propelled Swimming Microrobot Using Electroosmotic Propulsion and Biofuel Cell. IEEE Robot. Autom. Lett. 2018, 3, 1787–1792. [Google Scholar] [CrossRef]
- Jeon, S.; Ha, L.; Kim, S.; Kim, J.; Lee, K.; Choi, H.; Kim, D. Magnetically Actuated SiCN-Based Ceramic Microrobot for Guided Cell Delivery. Adv. Healthc. Mater. 2019, 8, 1900739. [Google Scholar] [CrossRef]
- Kim, D.; Abdolali, A.; Ahn, S. Patterned Magnetic Fields for Remote Steering and Wireless Powering to a Swimming Microrobot. IEEE/ASME Trans. Mechatron. 2020, 25, 207–216. [Google Scholar] [CrossRef]
- Jeon, S.; Nam, J.; Lee, W.; Jang, G. A Spiral Microrobot Performing Navigating Linear and Drilling Motions by Magnetic Gradient and Rotating Uniform Magnetic Field for Applications in Unclogging Blocked Human Blood Vessels. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Park, J.; Park, B.; Shin, Y.; Kim, K.; Park, H.H.; Ahn, S. Propulsion and Rotation of Microrobot Based on a Force on a Magnetic Material in a Time-Varying Magnetic Field Using a Wireless Power Transfer System. IEEE Trans. Magn. 2020, 56, 1–5. [Google Scholar] [CrossRef]
- Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Song, S.; He, P.; Li, H.; Mi, H.Y.; Wei, W.; Li, Z.; Xiong, X.; Li, Y. Motion Control of Magnetic Microrobot Using Uniform Magnetic Field. IEEE Access 2020, 8, 71083–71092. [Google Scholar] [CrossRef]
- Guo, S.; Yamauchi, Y.; Hirata, H.; Ishihara, H. A novel hybrid microrobot using rotational magnetic field for medical applications. Biomed. Microdevices 2015, 17, 31. [Google Scholar] [CrossRef]
- Tabak, A.F.; Hosney, A.; Mohamed, A.; Klingner, A.; Ghoneima, M.; Sitti, M. Sperm-shaped magnetic microrobots: Fabrication using electrospinning, modeling, and characterization. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 1939–1944. [Google Scholar] [CrossRef]
- Hirano, T.; Shibata, T. Phototactic algae-driven unidirectional transport of submillimeter-sized cargo in a microchannel. Micromachines 2019, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Ahn, S. Laser Controlled 65 Micrometer Long Microrobot Made of Ni-Ti Shape Memory Alloy. Adv. Mater. Technol. 2019, 4, 1900583. [Google Scholar] [CrossRef]
- Glückstad, J. Gearing up for optical microrobotics: Micromanipulation and actuation of synthetic microstructures by optical forces. Laser Photonics Rev. 2013, 7, 478–494. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Ohta, A.T. Interactive actuation of multiple opto-thermocapillary flow-addressed bubble microrobots. Robot. Biomim. 2014, 1, 14. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Xiong, Z.; Zhan, X.; Dai, W.; Li, C.C.; Feng, S.P.; Tang, J. Programmable artificial phototactic microswimmer. Nat. Nanotechnol. 2016, 11, 1087–1092. [Google Scholar] [CrossRef]
- Saadatzi, M.N.; Zhang, R.; Sherehiy, A.; Wei, D.; Harnett, C.K.; Popal, D.O. Multiphysics Dynamic Model Validation Methodology for Laser-Driven Microrobots. In Proceedings of the 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), Vancouver, BC, Canada, 22–26 August 2019; pp. 1555–1561. [Google Scholar] [CrossRef]
- Sherehiy, A.; Yang, Z.; Wei, D.; Harnett, C.K.; Popa, D.O. ChevBot—An Untethered Microrobot Powered by Laser for Microfactory Applications. In Proceedings of the 2019 International Conference on Robotics and Automation (ICRA), Montreal, QC, Canada, 20–24 May 2019; pp. 231–236. [Google Scholar] [CrossRef]
- Cortese, A.J.; Dorsey, K.; Esposito, E.P.; Reynolds, M.F.; Liu, Q.; Cao, M.; Muller, D.A.; McEuen, P.L.; Cohen, I. Electronically integrated, mass-manufactured, microscopic robots. Nature 2020, 584, 557–561. [Google Scholar] [CrossRef]
- Mark, A.G.; Reigh, S.Y.; Melde, K.; Qiu, T.; Zeng, H.; Parmeggiani, C.; Martella, D.; Sanchez-Castillo, A.; Kapernaum, N.; Giesselmann, F.; et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Bai, L.; Gentekos, D.T.; Hoyos, M.; Mallouk, T.E. Density and Shape Effects in the Acoustic Propulsion of Bimetallic Nanorod Motors. ACS Nano 2016, 10, 4763–4769. [Google Scholar] [CrossRef]
- Orozco, J.; Sattayasamitsathit, S.; Soto, F.; Kuralay, F.; Pourazary, A.; Katzenberg, A.; Gao, W.; Shen, Y.; Wang, J. Functionalized Ultrasound-Propelled Magnetically Guided Nanomotors: Toward Practical Biomedical Applications. ACS Nano 2013, 7, 9232–9240. [Google Scholar] [CrossRef]
- Mahdy, D.; Sharkawy, A.e.; Moustafa, R.R.; Tabak, A.F.; Mitwally, M.E.; Hesham, S.; Hamdi, N.; Klingner, A.; Mohamed, A.; Sitti, M. Mechanical Rubbing of Blood Clots Using Helical Robots Under Ultrasound Guidance. IEEE Robot. Autom. Lett. 2018, 3, 1112–1119. [Google Scholar] [CrossRef]
- Xu, L.P.; Zhang, X. Ultrasound propulsion of micro-/nanomotors. Appl. Mater. Today 2017, 9, 493–503. [Google Scholar] [CrossRef]
- Valdez-Garduño, M.; Soto, F.; Garcia-Gradilla, V. Engineering Ultrasound Fields to Power Medical Micro/Nanorobots. Curr. Robot. Rep. 2021, 2, 21–32. [Google Scholar] [CrossRef]
- Yasa, O.; Wrede, P.; Sitti, M. Acoustically powered surface-slipping mobile microrobots. Proc. Natl. Acad. Sci. USA 2020, 117, 3469–3477. [Google Scholar] [CrossRef] [Green Version]
- Ozcelik, A.; Nourhani, A.; Lammert, P.E.; Crespi, V.H.; Huang, T.J. Acoustic actuation of bioinspired microswimmers. Lab A Chip 2017, 17, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Shields, C.W.; Velev, O.D. Engineering of Self-Propelling Microbots and Microdevices Powered by Magnetic and Electric Fields. Adv. Funct. Mater. 2018, 28, 1705953. [Google Scholar] [CrossRef]
- Cayre, O.J.; Bazant, M.Z.; Velev, O.D. Induced-Charge Electrophoresis of Metallodielectric Particles. Phys. Rev. Lett. 2008, 100, 058302. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Zhao, H.; Wu, N. Inducing Propulsion of Colloidal Dimers by Breaking the Symmetry in Electrohydrodynamic Flow. Phys. Rev. Lett. 2015, 115, 208302. [Google Scholar] [CrossRef] [Green Version]
- Paunov, V.N.; Petsev, D.N.; Velev, O.D. Remotely powered self-propelling particles and micropumps based on miniature diodes. Nat. Mater. 2007, 6, 235–240. [Google Scholar] [CrossRef]
- Kuhn, A. Propulsion of Microobjects by Dynamic Bipolar Self-Regeneration. J. Am. Chem. Soc. 2010, 132, 15918–15919. [Google Scholar] [CrossRef]
- Manesh, K.M.; Hua, J.; Sattayasamitsathit, S.; Wang, J. Hybrid nanomotor: A catalytically/magnetically powered adaptive nanowire swimmer. Small 2011, 7, 2047–2051. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Cho, S.; Jeong, S.; Jin, Z.; Lee, C.; Ko, S.Y.; Park, J.O.; Park, S. A hybrid actuated microrobot using an electromagnetic field and flagellated bacteria for tumor-targeting therapy. Biotechnol. Bioeng. 2015, 112, 1623–1631. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, Z.; Sun, M.; Sen, A.; Mallouk, T.E. A tale of two forces: Simultaneous chemical and acoustic propulsion of bimetallic micromotors. Chem. Commun. 2015, 51, 1020–1023. [Google Scholar] [CrossRef]
- Ye, G.; Chen, B.; Liu, H. A dual-driven biomimetic microrobot based on optical and magnetic propulsion. J. Micromech. Microeng. 2021, 31, 035003. [Google Scholar] [CrossRef]
- Li, T.; Xu, T.; Kiristi, M.; Liu, W.; Wu, Z.; Wang, J. Magneto–Acoustic Hybrid Nanomotor. Nano Lett. 2015, 15, 4814–4821. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, C.; Chen, T.; Hu, C.; Sun, L. Kink and Delta Self-Actuating Platinum Micro-Robot. IEEE Trans. Nanotechnol. 2018, 17, 603–606. [Google Scholar] [CrossRef]
- Singh, A.V.; Ansari, M.H.D.; Mahajan, M.; Srivastava, S.; Kashyap, S.; Dwivedi, P.; Pandit, V.; Katha, U. Sperm Cell Driven Microrobots—Emerging Opportunities and Challenges for Biologically Inspired Robotic Design. Micromachines 2020, 11, 448. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Yin, L.; Zhang, Q.; Askarinam, N.; Mundaca-Uribe, R.; Tehrani, F.; Karshalev, E.; Gao, W.; Zhang, L.; et al. ACE2 Receptor-Modified Algae-Based Microrobot for Removal of SARS-CoV-2 in Wastewater. J. Am. Chem. Soc. 2021, 143, 12194–12201. [Google Scholar] [CrossRef] [PubMed]
- Glover, P.M. Interaction of MRI field gradients with the human body. Phys. Med. Biol. 2009, 54, R99–R115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, V.; Davids, M.; Schad, L.R.; Wald, L.L.; Guérin, B. Investigating cardiac stimulation limits of MRI gradient coils using electromagnetic and electrophysiological simulations in human and canine body models. Magn. Reson. Med. 2020, 85, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Panych, L.P.; Madore, B. The physics of MRI safety. J. Magn. Reson. Imaging 2017, 47, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Lauterbur, P.C. Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance. Nature 1973, 242, 190–191. [Google Scholar] [CrossRef]
- Puigmartí-Luis, J.; Bergeles, C.; Chen, X.; Pellicer, E.; Sort, J.; Počepcová, V.; Ferreira, A.; Nelson, B.J. Imaging Technologies for Biomedical Micro- and Nanoswimmers. Adv. Mater. Technol. 2019, 4, 1800575. [Google Scholar] [CrossRef] [Green Version]
- Cheriet, F.; Beaudoin, G.; Martel, S. MRI visualization of a single 15 μm navigable imaging agent and future microrobot. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 4355–4358. [Google Scholar] [CrossRef]
- Cheriet, F.; Martel, S. Accurate positioning of magnetic microparticles beyond the spatial resolution of clinical MRI scanners using susceptibility artifacts. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 2800–2803. [Google Scholar] [CrossRef]
- Dahmen, C.; Belharet, K.; Folio, D.; Ferreira, A.; Fatikow, S. MRI-based dynamic tracking of an untethered ferromagnetic microcapsule navigating in liquid. Int. J. Optomechatron. 2016, 10, 73–96. [Google Scholar] [CrossRef]
- Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.O.; Choi, E.; Kim, C.S. Localization and Actuation for MNPs Based on Magnetic Field-Free Point: Feasibility of Movable Electromagnetic Actuations. Micromachines 2020, 11, 1020. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Bang, D.; Park, J.O.; Choi, E. Optimization of Field-Free Point Position, Gradient Field and Ferromagnetic Polymer Ratio for Enhanced Navigation of Magnetically Controlled Polymer-Based Microrobots in Blood Vessel. Micromachines 2021, 12, 424. [Google Scholar] [CrossRef]
- Zhang, X.; Hoshiar, A.K.; Yoon, J. Real-Time Two-Dimensional Magnetic Particle Imaging for Electromagnetic Navigation in Targeted Drug Delivery. Sensors 2017, 17, 2050. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandro, B.; Fu, X. Optical Imaging Modalities for Biomedical Applications. IEEE Rev. Biomed. Eng. 2010, 3, 69–92. [Google Scholar] [CrossRef]
- Dong, D.; Lam, W.; Xing, L.; Wei, T.; Sun, D. Automated In Vivo Navigation of Magnetic-Driven Microrobots Using OCT Imaging Feedback. IEEE Trans. Biomed. Eng. 2020, 67, 2349–2358. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Chan, K.F.; Zhang, L. Automated Control of Magnetic Spore-Based Microrobot Using Fluorescence Imaging for Targeted Delivery With Cellular Resolution. IEEE Trans. Autom. Sci. Eng. 2020, 17, 490–501. [Google Scholar] [CrossRef]
- Xing, J.; Yin, T.; Li, S.; Xu, T.; Ma, A.; Chen, Z.; Luo, Y.; Lai, Z.; Lv, Y.; Pan, H.; et al. Sequential Magneto-Actuated and Optics-Triggered Biomicrorobots for Targeted Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2008262. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Koukourakis, N.; Wang, J.; Kuschmierz, R.; Radner, H.; Czarske, J.W.; Schmidt, O.G. Real-Time IR Tracking of Single Reflective Micromotors through Scattering Tissues. Adv. Funct. Mater. 2019, 29, 1905272. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.F.; Yuan, K.; Wang, Q.; Xia, X.; Yang, L.; Ko, H.; Wang, Y.X.J.; Sung, J.J.Y.; Chiu, P.W.Y.; Zhang, L. Endoscopy-assisted magnetic navigation of biohybrid soft microrobots with rapid endoluminal delivery and imaging. Sci. Robot. 2021, 6, eabd2813. [Google Scholar] [CrossRef]
- Bleck, J.S. Basic principles of ultrasonography and its relevance for internal medicine. Der Internist 2012, 53, 251–260. [Google Scholar] [CrossRef] [PubMed]
- J, J. Medical ultrasound imaging. Prog. Biophys. Mol. Biol. 2007, 93, 153–165. [Google Scholar] [CrossRef]
- Kremkau, F. Medical ultrasound systems. Interface Focus 2011, 1, 477–489. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.; Oren, E.; Yu, T.; Qiu, T. A Helical Microrobot with an Optimized Propeller-Shape for Propulsion in Viscoelastic Biological Media. Robotics 2019, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.; Adam, G.; Lambert, E.; Solorio, L.; Goergen, C.J.; Cappelleri, D.J. A Tumbling Magnetic Microrobot System for Biomedical Applications. Micromachines 2020, 11, 861. [Google Scholar] [CrossRef]
- Niehoff, D.; Mohanty, S.; Misra, S. A Contactless and Biocompatible Approach for 3D Active Microrobotic Targeted Drug Delivery. Micromachines 2019, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yu, J.; Vong, C.I.; Yan Chiu, P.W.; Zhang, L. Magnetic Navigation of a Rotating Colloidal Swarm Using Ultrasound Images. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 5380–5385. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.F.; Schweizer, K.; Du, X.; Jin, D.; Yu, S.C.H.; Nelson, B.J.; Zhang, L. Ultrasound Doppler-guided real-time navigation of a magnetic microswarm for active endovascular delivery. Sci. Adv. 2021, 7, eabe5914. [Google Scholar] [CrossRef]
- Magdanz, V.; Schmidt, O.G.; Misra, S. Magnetic control of self-propelled microjets under ultrasound image guidance. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Sao Paulo, Brazil, 12–15 August 2014; pp. 169–174. [Google Scholar] [CrossRef]
- Sutherland, A. Molecular tracers for the PET and SPECT imaging of disease. Chem. Soc. Rev. 2011, 40, 149–162. [Google Scholar] [CrossRef]
- Cossío, U.; Parmar, J.; Martínez-Villacorta, A.M.; Gómez-Vallejo, V.; Llop, J.; Sánchez, S. Medical Imaging for the Tracking of Micromotors. ACS Nano 2018, 12, 1220–1227. [Google Scholar] [CrossRef]
- Go, G.; Jin, Z.; Darmawan, B.A.; Yoo, A.; Kim, S.; Nan, M.; Lee, S.B.; Kang, B.; Kim, C.; Li, H.; et al. A Magnetically Guided Self-Rolled Microrobot for Targeted Drug Delivery, Real-Time X-Ray Imaging, and Microrobot Retrieval. Adv. Healthc. Mater. 2021, 10, 2001681. [Google Scholar] [CrossRef]
- Balasundaram, G.; Moothanchery, M.; Dinish, U.; Bi, R.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef]
- Pramanik, M. Recent advances in photoacoustic contrast agents for in vivo imaging. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef]
- Jing, W.; Mehrmohammadi, M. Photoacoustic Imaging to Track Magnetic-manipulated Micro-Robots in Deep Tissue. Sensors 2020, 20, 2816. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Jing, W. Non-invasive Photoacoustic Imaging of Magnetic Microrobot through Deep Non-Transparent Tissue. In Proceedings of the 2020 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Toronto, ON, Canada, 13–17 July 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Pang, X.; Yan, X.; Dai, Q.; Lin, H.; Ye, J.; Cheng, Y.; Zhao, Q.; Ma, X.; Zhang, X.; Liu, G.; et al. Photoacoustic Imaging-Trackable Magnetic Microswimmers for Pathogenic Bacterial Infection Treatment. ACS Nano 2020, 14, 2880–2893. [Google Scholar] [CrossRef]
- Mahgerefteh, S.Y.; Sosna, J.; Goldberg, S.N. Image-Guided Fusion and Navigation: Applications in Tumor Ablation. Tech. Vasc. Interv. Radiol. 2013, 16, 287–295. [Google Scholar] [CrossRef]
- Cova, L.; de Beni, S.; Ierace, T.; Tondolo, T.; Cerri, A.; Goldberg, S.N.; Solbiati, L. Real-Time US-CT/MRI Image Fusion for Guidance of Thermal Ablation of Liver Tumors Undetectable with US: Results in 295 Cases. Cardio Vasc. Interv. Radiol. 2015, 38, 143–151. [Google Scholar] [CrossRef]
- Paul-Gilloteaux, P.; Plochberger, B.; Sefc, L.; Verkade, P.; Mannheim, J.G.; Slezak, P.; Unterhuber, A.; Marchetti-Deschmann, M.; Ogris, M.; Bühler, K.; et al. Correlated Multimodal Imaging in Life Sciences: Expanding the Biomedical Horizon. Front. Phys. 2020, 8, 47. [Google Scholar] [CrossRef]
- Li, Y.; Nguyen, V.P.; Huang, Z.; Liu, Z.; Wang, X.; Paulus, Y.M. High-resolution, in vivo multimodal photoacoustic microscopy, optical coherence tomography, and fluorescence microscopy imaging of rabbit retinal neovascularization. Light. Sci. Appl. 2018, 7, 103. [Google Scholar] [CrossRef] [Green Version]
- Jing, W.; Mehrmohammadi, M. Submillimeter Magnetic Microrobot Tracking Using an Integrated Ultrasound and Photoacoustic Imaging System. In Proceedings of the 2019 IEEE International Ultrasonics Symposium (IUS), Glasgow, UK, 6–9 October 2019; pp. 1057–1060. [Google Scholar] [CrossRef]
- Chitgupi, U.; Lovell, J.F. Recent Advances in Higher-Order, Multimodal, Biomedical Imaging Agents. Small 2015, 11, 4445–4461. [Google Scholar] [CrossRef]
- von Gladiss, A.; Friedrich, T.; Heinen, U.; Lehr, H.; Lüdtke-Buzug, K.; Buzug, T.M. Actuation and visualization of a magnetically coated swimmer with magnetic particle imaging. J. Magn. Magn. Mater. 2019, 473, 495–500. [Google Scholar] [CrossRef]
- Jakab, P.; Székely, G.; Hata, N. MRI driven magnetic microswimmers. Biomed. Microdevices 2012, 14, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Rahmer, J.; Gleich, B.; Halkola, A.; Buzug, T.M.; Borgert, J. Steering of Magnetic Devices With a Magnetic Particle Imaging System. IEEE Trans. Biomed. Eng. 2016, 63, 2286–2293. [Google Scholar] [CrossRef]
- Lalande, V.; Bringout, G.; Tremblay, C.; Martel, S. A MRI-based integrated platform for the navigation of microdevices and microrobots. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 1285–1290. [Google Scholar] [CrossRef]
- Folio, D.; Ferreira, A. MRI-based microrobotic system for the propulsion and navigation of ferromagnetic microcapsules. Minim. Invasive Ther. Allied Technol. 2010, 19, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Ludewig, P.; Thieben, F.; Gdaniec, N.; Knopp, T. Imaging and moving magnetic beads with magnetic particle imaging for targeted drug delivery. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 1293–1296. [Google Scholar] [CrossRef]
- Chen, M.; Lee, H.; Feng, S.P.; Park, J.Y.; Lee, S.; Kim, J.T. X-ray-Powered Micromotors. ACS Appl. Mater. Interfaces 2019, 11, 15727–15732. [Google Scholar] [CrossRef]
- Park, J.O.; Park, S.; Ko, S.Y. Image-based guidance system for intravascular microrobot: Fiducial marker-based registration using biplanar fluoroscopic images & CTA images. In Proceedings of the 2015 15th International Conference on Control, Automation and Systems (ICCAS), Busan, Korea, 13–16 October 2015; pp. 919–922. [Google Scholar] [CrossRef]
- Zhou, Q.; Vincent, M.; Deng, Y.; Yu, J.; Xu, J.; Xu, T.; Tang, T.; Bian, L.; Wang, Y.X.J.; Kostarelos, K.; et al. Multifunctional biohybrid magnetite microrobots for imaging-guided therapy. Sci. Robot. 2017, 2, eaaq1155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Yang, L.; Vong, C.I.; Chan, K.F.; Wu, W.K.K.; Kwong, T.N.Y.; Lo, N.W.S.; Ip, M.; Wong, S.H.; Sung, J.J.Y.; et al. Real-time tracking of fluorescent magnetic spore–based microrobots for remote detection of C. diff toxins. Sci. Adv. 2019, 5, eaau9650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, D.; Chen, S.; Zhang, Y.; Li, J.; Fan, L.; Guan, Z.; Lo, C.; Wang, L.; Man, K.; et al. Development of Magnet-Driven and Image-Guided Degradable Microrobots for the Precise Delivery of Engineered Stem Cells for Cancer Therapy. Small 2020, 16, 1906908. [Google Scholar] [CrossRef]
- Chrostowski, R. Frontiers of Medical Micro/Nanorobotics: In vivo Applications and Commercialization Perspectives Toward Clinical Uses. Front. Bioeng. Biotechnol. 2018, 6, 170. [Google Scholar] [CrossRef]
- de Momi, E.; el Hadji, S.; Mattos, L.S. Blood vessel segmentation algorithms—Review of methods, datasets and evaluation metrics. Comput. Methods Programs Biomed. 2018, 158, 71–91. [Google Scholar] [CrossRef] [Green Version]
- Folio, D.; Ferreira, A. 3D MRI-based predictive control of a ferromagnetic microrobot navigating in blood vessels. In Proceedings of the 2010 3rd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Tokyo, Japan, 26–29 September 2010; pp. 808–813. [Google Scholar] [CrossRef] [Green Version]
- Folio, D.; Ferreira, A. Endovascular navigation of a ferromagnetic microrobot using MRI-based predictive control. In Proceedings of the 2010 IEEE/RSJ International Conference on Intelligent Robots and Systems, Taipei, Taiwan, 18–22 October 2010; pp. 2804–2809. [Google Scholar] [CrossRef] [Green Version]
- Sohn, S.W.; Lee, H.; Choi, D.; Jang, E.; Kim, M.; Lee, J.; Park, S. Analysis and Evaluation of Path Planning Algorithms for Autonomous Driving of Electromagnetically Actuated Microrobot. Int. J. Control Autom. Syst. 2020, 18, 2943–2954. [Google Scholar] [CrossRef]
- Misra, S. An experimental comparison of path planning techniques applied to micro-sized magnetic agents. In Proceedings of the 2016 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Paris, France, 18–22 July 2016; pp. 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rogowski, L.; Kim, M.J.; Becker, A.T. Path planning and aggregation for a microrobot swarm in vascular networks using a global input. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 414–420. [Google Scholar] [CrossRef]
- Nguyen, P.B.; Kang, B.; Bappy, D.M.; Choi, E.; Park, S.; Ko, S.Y.; Park, J.O.; Kim, C.S. Real-time microrobot posture recognition via biplane X-ray imaging system for external electromagnetic actuation. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 1843–1852. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Gong, Z.; Holmes, G.; Chen, Y. A Feasibility Study of In Vivo Control and Tracking of Microrobot Using Taxicab Geometry for Direct Drug Targeting. IEEE Trans. NanoBioscience 2021, 20, 235–245. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, S.; Liu, L. Instantaneous Velocity Estimation of Magnetic Microrobots with Visual Tracking. In Proceedings of the 2019 IEEE International Conference on Robotics and Biomimetics (ROBIO), Dali, China, 6–8 December 2019; pp. 1508–1512. [Google Scholar] [CrossRef]
- Yu, J.; Yan, X.; Choi, H.; Zhang, L. Magnetic Actuation Based Motion Control for Microrobots: An Overview. Micromachines 2015, 6, 1346–1364. [Google Scholar] [CrossRef]
- Jeong, S.; Cha, K.; Qin, L.; Li, J.; Park, J.; Park, S. Positioning of microrobot in a pulsating flow using EMA system. In Proceedings of the 2010 3rd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Tokyo, Japan, 26–29 September 2010; pp. 588–593. [Google Scholar] [CrossRef]
- Go, G.; Lee, C.; Ko, S.Y.; Jeong, S.; Kwon, K.; Park, J.O.; Park, S. Electromagnetic actuation system for locomotive intravascular therapeutic microrobot. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, Sao Paulo, Brazil, 12–15 August 2014; pp. 831–834. [Google Scholar] [CrossRef]
- Decanini, D.; Hwang, G. The Rotation of Microrobot Simplifies 3D Control Inside Microchannels. Sci. Rep. 2018, 8, 438. [Google Scholar] [CrossRef]
- Zhang, L. External Power-Driven Microrobotic Swarm: From Fundamental Understanding to Imaging-Guided Delivery. ACS Nano 2021, 15, 149–174. [Google Scholar] [CrossRef]
- Schmidt, O.G. Medical microbots need better imaging and control. Nature 2017, 545, 406–408. [Google Scholar] [CrossRef] [Green Version]
- Katsanos, K.; Karnabatidis, D.; Loudos, G.; Nikiforidis, G.C.; Hendee, W.R. Emerging technologies for image guidance and device navigation in interventional radiology. Med. Phys. 2012, 39, 5768–5781. [Google Scholar] [CrossRef]
- Kawamura, K.; Inoue, F.; Ikuta, K.; Ikeuchi, M. Magnetically Controlled Microrobot for Embryo Transfer in Assisted Reproductive Technology. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (TRANSDUCERS & EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 2217–2220. [Google Scholar] [CrossRef]











| Actuation Method | Actuation Equipment | Microrobot’s Feature Materials/ Structural | Microrobot’s Feature Size | Ref |
|---|---|---|---|---|
| Magnetic field | Three orthogonal electromagnetic coils | Nano-helix robot: InGaAs/GaAs/Cr helical tail and Cr/Ni/Au soft-magnetic head | Diameter 2.8 m | [15] |
| Magnetic field | 3 pairs of Maxwell coils and 3 pairs of Helmholtz coils | Cylindrical NdFeB (N42) magnet | 2 mm in diameter and 3 mm in height | [27] |
| Magnetic field | Distributed diagonally in eight electromagnetic coils with DT4 cores | Patterned seaweed hydrogel with PEGDA magnetic microspheres | −500 m | [16] |
| Light field | A green LED (emission maximum centered at = 525 nm, outer diameter: 3 mm, Stanley Electric, UG3803X, Tokyo, Japan) | Phototactic algae | [30] | |
| Light field | Commercial optical tweezers (Elliot Scientific, E3500) | Hinged microrobot | −20 m | [11] |
| Light field | 808-nm laser with focus size of 354 m | Au coating | 45 m | [13] |
| Light field | 532 nm laser | Si | 520 × 260 m | [36] |
| Light field | Pulsed laser | Si/Pt | 40 × 70 m | [37] |
| Acoustics | Piezoelectric transducer | Au, Rh, Pd, Ag, Pt, and Ru metals | −1 m | [39] |
| Electric field | Two electrodes diode | mm level | [49] | |
| Hybrid actuation | Three-axis Helmholtz coil | Multi-segment Pt-Au-- Ni nanowires | 10m | [51] |
| Hybrid actuation | Infrared lasers and electromagnetic coils | Ni/Ain | −100 m | [54] |
| Hybrid actuation | Electromagnetic coils and piezoelectric transducer | Nickel-plated gold nanorod and nickel-plated niobium nano helix | −50 m | [55] |
| Imaging Method | Microrobot’s Feature Material | Microrobot’s Feature Size | Ref |
|---|---|---|---|
| MRI | Magnetic particles | −15 m | [64,65] |
| MPI | Resovist particles | 45 to 65 nm | [70] |
| OCT | Diameter 90 m | [72] | |
| Fluorescence imaging | Carbon point | [73] | |
| Light reflection imaging | Silicon dioxide and a thin gold layer | 100 and 20 microns | [75] |
| US | Rotate the colloidal group | A single 500 nm | [83] |
| US | Platinum microtubes | [85] | |
| PET | Au/ Iodine isotope | −12 m | [87] |
| CT | X-ray contrast agent | Radius 250 m | [88] |
| PAI | Ni particles | >50 m | [92] |
| Method | Actuation | Medical Imaging |
|---|---|---|
| Magnetic | High penetration depth; good actuation and control performance;safe; decays with distance | MRI: High penetration depth; good resolution; long imaging time. MPI: Fast imaging; limited field of view |
| Light | Low penetration depth; high spatial selectivity, which can be decentralized to control multiple microrobots; | High resolution; poor penetration depth |
| Acoustic | High penetration depth; safe; no special requirements for microrobot’s materials; heat generation when actuating | Fast imaging; poor resolution |
| Electric | Low cost; high-ion media required | |
| Radiation | High penetration depth; high resolution; long imaging time; hazardous | |
| PA | Fast imaging; high resolution; improved penetration depth | |
| Hybrid Actuation/ Multimodal Imaging | Higher actuation and control performance | Multi-dimensional information; better localizing performance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Li, C.; Dong, L.; Zhao, J. A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo. Micromachines 2021, 12, 1249. https://doi.org/10.3390/mi12101249
Li Z, Li C, Dong L, Zhao J. A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo. Micromachines. 2021; 12(10):1249. https://doi.org/10.3390/mi12101249
Chicago/Turabian StyleLi, Zhongyi, Chunyang Li, Lixin Dong, and Jing Zhao. 2021. "A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo" Micromachines 12, no. 10: 1249. https://doi.org/10.3390/mi12101249
APA StyleLi, Z., Li, C., Dong, L., & Zhao, J. (2021). A Review of Microrobot’s System: Towards System Integration for Autonomous Actuation In Vivo. Micromachines, 12(10), 1249. https://doi.org/10.3390/mi12101249