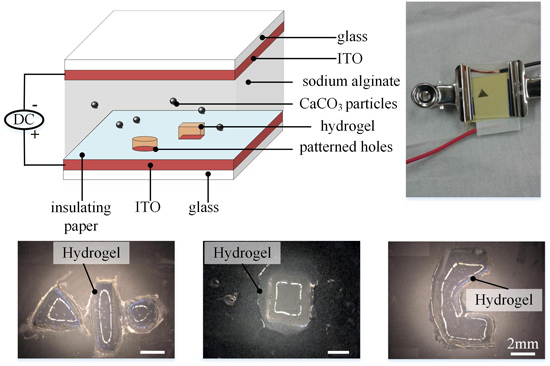
3.1. Control of 3D Hydrogel Patterns through Insulating Paper
Electrodes of quadrangle shape are firstly demonstrated to generate hydrogels. The patterned quadrangle hole is 5 mm × 5 mm in size. The concentrations for sodium alginate and CaCO
3 are 1%
w/
v and 0.25%
w/
v, respectively. 3 V DC potential stood for 12 min before being switched off. Here, a long time, 12 min, is used to achieve hydrogel of large height for easy observation. As we can see later, hydrogel of several hundred micrometers can form in just 1 min, and this time can be reduced by applying a larger DC potential. After that, the top ITO glass was removed. The bottom ITO glass and formed hydrogel on it were rinsed softly with NaCl solution (1%
w/
v). After being rinsed, the hydrogel was observed with an optical microscope (VHX-1000, Keyence, Japan) and are shown in
Figure 3. The four pictures are imaged at different observation angle,
i.e., 0°, 30°, 60° and 90°.
As we can see in
Figure 3, calcium alginate hydrogel only forms on patterned electrodes and hydrogel has the same shape as patterned electrodes. The results have demonstrated that the pattern of hydrogel has been successfully controlled by pattern of electrodes. This is because when a DC potential is applied, only the patterned holes on the bottom ITO glass would electrolyze water and generate hydrogen ions H
+ and calcium ions Ca
2+. According to Equation (3), Ca
2+ ions are required in the process of generating calcium hydrogel. Yet, Ca
2+ ions cannot be generated elsewhere except near electrodes, so hydrogel only forms on patterned holes and has the same shape as patterned holes.
Figure 3.
Hydrogel of quadrangle shape observed from (a) 0°; (b) 30°; (c) 60° and (d) 90°. Hydrogel is framed by red dashed line. The scale bar is 2 mm.
Figure 3.
Hydrogel of quadrangle shape observed from (a) 0°; (b) 30°; (c) 60° and (d) 90°. Hydrogel is framed by red dashed line. The scale bar is 2 mm.
In addition to the quadrangle shape, hydrogels with other shapes can also be fabricated by fabricating different shapes on insulated paper. As illustrated in
Figure 4, we prepared the paper pattern with four various shapes, including circle, triangle, rectangle and a “C” letter, and generated the gel following the same electrodeposition process. The results in
Figure 4 prove that the gel structure can be controlled easily by designing the paper’s pattern.
Figure 4.
(a) Top view and (b) side view of hydrogel of triangle shape, rectangle shape and circle shape on one electrodeposition chip. (c) Top view and (d) side view of hydrogel of letter “C” shape. Hydrogel is framed by red dashed line. The scale bar is 2 mm.
Figure 4.
(a) Top view and (b) side view of hydrogel of triangle shape, rectangle shape and circle shape on one electrodeposition chip. (c) Top view and (d) side view of hydrogel of letter “C” shape. Hydrogel is framed by red dashed line. The scale bar is 2 mm.
Moreover, this method is also effective for the gel generation with small size. To demonstrate that, a hole of 500 µm is punched with a needle on the paper (
Figure 5a) firstly. Considering the alginate hydrogel is a highly water-contained (~98%) material, the gel with 500 µm size is very difficult to observe by optical microscope directly due to the gel’s low contrast to water. Therefore, 2 mg fluorescein 5(6)-isothiocyanate (Sigma, USA) is added into 10 mL sodium alginate solution, and the generated hydrogel is observed with a fluorescence microscope (NI-S-E, Nikon, Tokyo, Japan). As the results shown in
Figure 5b, a circular hydrogel of 500 µm in diameter is achieved with accurate contour.
The above experiment results verify that this paper-based electrodeposition chip is able to control the pattern of the alginate hydrogel easily and simply. Hydrogel’s shape and size follow the holes’ shape and size. As a result, hydrogel of different patterns can be achieved by fabricating different patterns on the insulating paper.
Figure 5.
(a) Punched hole of 500 µm in diameter and (b) corresponding generated hydrogel observed with fluorescence microscope.
Figure 5.
(a) Punched hole of 500 µm in diameter and (b) corresponding generated hydrogel observed with fluorescence microscope.
3.2. Control of 3D Hydrogel’s Growth
In the previous section, hydrogel’s shapes have been demonstrated to be controllable. In this section, experiments are presented to control hydrogel’s growth,
i.e., hydrogel’s height. The experimental setup is shown in
Figure 1. In the experiments, the size of patterned holes is 5 mm × 5 mm. The effect of both DC potential and reaction time have been studied. Three different potentials (3.0 V, 3.2 V, 3.4 V) are used. For each potential, hydrogel’s height for different reaction time are measured. The reaction time is chosen to be 1, 2, 3 and 5 min. DC currents during electrodeposition are also measured.
Figure 6 shows time-dependent height of hydrogel for different potentials. In addition to height, the electrodeposition current has also been measured. For each of the three potentials, electrodeposition currents (
Figure 7) have been measured for three times. As was expected, longer reaction time leads to higher hydrogel. For a longer time, more H
+ would be generated, and therefore more Ca
2+ and calcium alginate hydrogel would be generated, so height of hydrogel would increase as time processes. However, the increase rate of hydrogel’s height decreases as time goes on. The first reason for decreasing rate is that as reaction goes on, the concentration of sodium alginate and CaCO
3 particles decreases, and then the formation speed of calcium hydrogel would decrease accordingly. The second reason is that some generated hydrogel does not continue to contribute to the hydrogel’s height increase, but instead contributes to the hydrogel’s density increase.
Figure 6.
Height of hydrogel for different reaction time and for different DC potential. As time goes on, the concentrations of sodium alginate and CaCO3 particles decrease, which then makes the rate of Ca2+ and hydrogel’s formation decrease. As a result, the increasing rate of hydrogel’s height decreases. The size of patterned electrode is 5 mm × 5 mm.
Figure 6.
Height of hydrogel for different reaction time and for different DC potential. As time goes on, the concentrations of sodium alginate and CaCO3 particles decrease, which then makes the rate of Ca2+ and hydrogel’s formation decrease. As a result, the increasing rate of hydrogel’s height decreases. The size of patterned electrode is 5 mm × 5 mm.
Figure 7.
Electrodeposition current for different potential. For each of the three potentials, currents were measured three times. The three measured currents match each other well, which means that the repeatability of proposed 3D hydrogel patterning system is high. The used electrode is 5 mm × 5 mm in size.
Figure 7.
Electrodeposition current for different potential. For each of the three potentials, currents were measured three times. The three measured currents match each other well, which means that the repeatability of proposed 3D hydrogel patterning system is high. The used electrode is 5 mm × 5 mm in size.
As shown in
Figure 7, the three currents for each potential match each other very well and the differences between the three currents are negligible. It means the repeatability of this 3D cell patterning system is high. From these curves, we can see that the deposition current is at their maximums in the beginning, decrease quickly at the first few seconds, and finally keep stable. The reason for the current’s decrease is that the concentration of sodium alginate and CaCO
3 particles is highest at the beginning and at this stage, it is easy for generated H
+ to react with CaCO
3 particles. Later, as the concentration of sodium alginate and CaCO
3 particles decrease, it will become more and more difficult for H
+ to react with CaCO
3 particles. The decreasing rate of H
+ consumption would slow down the process of water’s electrolysis. Thus, the electrodeposition current decreases as time goes on.
3.3. Equivalent Electric Circuit of the Electrodeposition System
Figure 8 gives the electrodeposition system’s equivalent electric circuit. The voltage source
V is the applied electrochemical potential.
ED is counter potential. This counter potential includes solution’s hydrolysis potential and contact potential drop. Solution’s hydrolysis potential is the minimum potential required to electrolyze solution. The contact potential drop is caused by Warburg elements and contact resistance between wires and ITO plate. When the voltage source
V is smaller than counter potential
ED, chemical reaction wouldn’t happen.
C1 represents the capacitor between two plate electrodes,
i.e., two pieces of ITO glass.
C1 is decided by the ITO glass’s size, the vertical distance between the two ITO glasses and sodium alginate solution’s permittivity.
C2 represents the double layer capacitor between ITO surface and solution and is caused by electrical double layer effect.
C2 appears at the interface between ITO glass’s surface and the solution adjacent to ITO glass’s surface, and is decided by solution’s permittivity and thickness of separation layer. Resistor
R1 is the equivalent series resistance of
C1. Resistor
R2 includes the equivalent series resistance of
C2 and solution’s resistance. Resistor
R3 includes power supply’s internal resistance and wires’ resistance. Unlike usual electrodeposition system with rod electrodes, a capacitor (
C1) should be added to represent the plate electrodes’ capacitance.
According to
Figure 8, the following equations can be achieved:
where
U1 and
U2 are capacitor
C1 and
C2’s voltage respectively.
Figure 8.
The equivalent circuit of the electrodeposition system for hydrogel. V is the applied voltage. ED is counter potential, includes solution’s hydrolysis potential and contact potential drop. C1 represents the capacitor between two plate electrodes. C2 represents the double layer capacitor between ITO surface and solution. Resistor R1 is the equivalent series resistance of C1. Resistor R2 includes the equivalent series resistance of C2 and solution’s resistance. Resistor R3 includes power supply’s internal resistance and wires’ resistance.
Figure 8.
The equivalent circuit of the electrodeposition system for hydrogel. V is the applied voltage. ED is counter potential, includes solution’s hydrolysis potential and contact potential drop. C1 represents the capacitor between two plate electrodes. C2 represents the double layer capacitor between ITO surface and solution. Resistor R1 is the equivalent series resistance of C1. Resistor R2 includes the equivalent series resistance of C2 and solution’s resistance. Resistor R3 includes power supply’s internal resistance and wires’ resistance.
As we can see in Equation (4), because of
R3, current
I1 and
I2 are coupled together. In order to simplify the calculation and be able to derive an analytical solution/expression for Equation (4),
R3 is set to be zero.
R3 represents the sum of power supply’s internal resistance and wires’ resistance, so its value is small. In addition, as we can see in
Figure 9, the deposition current decreases as time goes on. As a result, setting
R3 to be zero would have less and less effect on the accuracy of the built equivalent circuit. After
R3 has been set to be zero, the analytical solution for Equation (4) can be given by the following equation:
Figure 9.
Measured electrodeposition currents and predicted currents by equivalent circuit for different applied potential. The mean absolute differences between measured currents and predicted currents for DC potentials 3.0, 3.2 and 3.4 V are 0.1776, 0.3037 and 0.5776 A/m2, respectively. The results demonstrate that the predicated currents and measured currents match well. Thus, the built equivalent circuit is justified.
Figure 9.
Measured electrodeposition currents and predicted currents by equivalent circuit for different applied potential. The mean absolute differences between measured currents and predicted currents for DC potentials 3.0, 3.2 and 3.4 V are 0.1776, 0.3037 and 0.5776 A/m2, respectively. The results demonstrate that the predicated currents and measured currents match well. Thus, the built equivalent circuit is justified.
Following the process of building an equivalent circuit for the electrodeposition system and deriving an analytical solution for the equivalent circuit, the next step is to calculate parameters in the analytical solution,
i.e., Equation (5). Those parameters that need to be calculated include counter potential
ED, capacitor
C1 and
C2, resistor
R1 and
R2. After those parameters have been computed, the correctness should be tested. In
Figure 9, electrodeposition current for three different DC potentials have been measured. Electrodeposition currents for potential 3.4 and 3.2 V will be used to compute parameters and electrodeposition currents for potential 3.0 V will be used to justify the correctness of parameters computation.
Counter potential
ED is first computed. When DC potential is initially applied to the electrodeposition cell, the capacitor can be seen as short-circuited. Thus, the equivalent circuit in
Figure 8 can be regarded as a circuit only with resistors at the beginning of applying DC potential. This means that the ratio of applied potential minus counter potential to initial electrodeposition current is a constant, which is the equivalent resistance of the built circuit at the instant DC potential is applied. Thereby, the following equation can be achieved:
where applied potential
V2,
V3 are 3.2 and 3.4 V, respectively;
Jd2_0 and
Jd3_0 are the initial electrodeposition current density for potential 3.2 and 3.4 V, respectively;
S is the anode’s area. Then, the counter potential
ED can be achieved as following:
Next, the electrodeposition current for potential 3.2 and 3.4 V are processed with Matlab’s curve fitting box, and the two currents are expressed as the following equations:
According to Equations (8) and (9), two set of those four parameters: capacitor
C1 and
C2, resistor
R1 and
R2, can be achieved, and their values are determined through arithmetic mean value of the two set of results. The results are shown in
Table 1. Therefore, the decomposition current can be expressed as:
After the expression for electrodeposition current is achieved, their validity can be examined by compared the current got by theory with the current got by experiments.
Figure 9 gives the current given by the built equivalent circuit and the current measured during experiments. The mean absolute differences ɛ
3.0, ɛ
3.2, ɛ
3.4 between measured currents and predicted currents for DC potentials 3.0, 3.2 and 3.4 V are:
The results in
Figure 9 show that currents by built equivalent circuit and currents by experiments match each other well. This good match justifies the equivalent circuit. Although the overall match between predicted currents and measured currents is very good, the initial difference between them is relatively large. One reason is that resistor
R3 is set to be zero to decouple the two differential equations related to the two capacitors in
Figure 8, respectively. In addition, other elements, e.g., inductor, affecting the transient state of the equivalent circuit are not considered in the equivalent circuit, which would also result in initial errors.
Table 1.
Parameters achieved by curve fitting and the final decided value for parameters in built circuit model.
Table 1.
Parameters achieved by curve fitting and the final decided value for parameters in built circuit model.
| Parameter | R1 (Ω) | C1 (F) | R2 (Ω) | C2 (F) |
|---|
| Group 1 | 5758 | 6.612 × 10−2 | 3335 | 6.442 × 10−3 |
| Group 2 | 4609 | 9.034 × 10−2 | 3407 | 5.548 × 10−3 |
| Decided value | 5184 | 7.823 × 10−2 | 3371 | 5.995 × 10−3 |
3.4. Hydrogel’s Growth Model
As is shown in Equations (1)–(3), the generated mass of hydrogel is proportional to generated number H
+. Because generated number of H
+ is proportional to the number of electric charges, then the mass of hydrogel is proportional to the number of electric charges, which can be expressed as the following equation:
where ρ is hydrogel’s density;
S is hydrogel’s area and
h is hydrogel’s height;
l is a constant and
Q is electric charges;
J is current density. Therefore:
Equation (10) gives the electrodeposition current, which can be divided into two charging currents of capacitors. However, only one charging current contributes to hydrogel’s formation. Capacitor
C1’s charging current wouldn’t go through solution, as charges would gather on the two ITO glasses and wouldn’t go through the solution between the two ITO glasses. Thus, only capacitor
C2’s charging current contributes Hydrogel’s formation. Then hydrogel’s expression can be achieved by substituting
C2’s charging current to Equation (15), and hydrogel’s height can be expressed as the following equation:
where
k is constant that need to be calculated and
V is electrochemical potential.
Next, hydrogel’s height for potential 3.2 and 3.4 V are processed with Matlab’s curve fitting box. The calculate
k for these two potentials are given in
Table 2. The final
k is chosen as the mean value of the two calculated
k.
Table 2.
Constants computed by experiments with 3.2 V and 3.4 V and final mean value for the constant.
Table 2.
Constants computed by experiments with 3.2 V and 3.4 V and final mean value for the constant.
| Voltage | 3.2 V | 3.4 V | Mean Value |
|---|
| k | 1686 | 1765 | 1725.5 |
Then hydrogel’s height can be given in the following equation:
Figure 10 gives hydrogel’s height given by growth model and hydrogel’s height measured during experiments. Overall, hydrogel’s growth model can predict hydrogel growth well. One reason for errors between model and experiments is that during deducing Equation (14), we assume that hydrogel’s height over the area is the same. However, hydrogel’s height over the area is not the same with hydrogel’s center being highest.
Figure 10.
Hydrogel’s height given by hydrogel’s growth model and measured during experiments. The results demonstrate that hydrogel’s growth model can reflect the overall change of hydrogel’s height over time.
Figure 10.
Hydrogel’s height given by hydrogel’s growth model and measured during experiments. The results demonstrate that hydrogel’s growth model can reflect the overall change of hydrogel’s height over time.