Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices
Abstract
:1. Background
2. Case-Study: Barriers to Adoption of Low-Cost RDTs in Kenya
3. Results and Discussion
4. What Are the Main Barriers to Adopting RDTs?
5. Conclusions: What We Learned
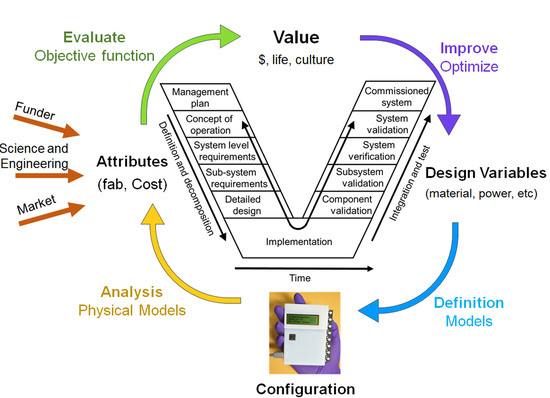
6. Outlook: New Design Paradigm
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Development Programme. Assessing Progress in Africa towards the Millennium Development Goals; United Nations Economic Commission for Africa: Addis Ababa, Ethiopia, 2010. [Google Scholar]
- Wamai, R.G. The Kenya health system—Analysis of the situation and enduring challenges. Japan Med. Assoc. J. 2009, 52, 134–140. [Google Scholar]
- Petti, C.A.; Polage, C.R.; Quinn, T.C.; Ronald, A.R.; Sande, M.A. Laboratory medicine in Africa: A barrier to effective health care. Clin. Infect. Dis. 2006, 42, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Bates, I.; Maitland, K. Are laboratory services coming of age in sub-Saharan Africa? Clin. Infect. Dis. 2006, 42, 383–384. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.A.; Hennek, J.W.; Smith, B.S.; Kumar, S.; Beattie, P.; Jain, S.; Rolland, J.P.; Stossel, T.P.; Chunda-Liyoka, C.; Whitesides, G.M. From the bench to the field in low-cost diagnostics: Two case studies. Angew. Chem. Int. Ed. 2015, 54, 5836–5853. [Google Scholar] [CrossRef] [PubMed]
- Oyola-Reynoso, O.; Tevis, I.D.; Chen, J.; Chang, B.S.; Cinar, S.; Bloch, J.-F.; Thuo, M. Recruiting Physi-sorbed Water in Surface Polymerization for Bio-Inspired Materials of Tunable Hydrophobicity. J. Mater. Chem. A 2016, 4, 14729–14738. [Google Scholar] [CrossRef]
- Oyola-Reynoso, S.; Frankiewicz, C.; Chang, B.S.; Chen, J.; Bloch, J.; Thuo, M.M. Paper-Based Microfluidic Devices by Asymmetric Calendering. Biomicrofluidics 2017, 11, 014104. [Google Scholar] [CrossRef] [PubMed]
- Oyola-Reynoso, S.; Heim, A.P.; Halbertsma-Black, J.; Zhao, C.; Tevis, I.D.; Cinar, S.; Cademartiri, R.; Liu, X.; Bloch, J.F.; Thuo, M.M. Draw your assay: Fabrication of low-cost paper-based diagnostic and multi-well test zones by drawing on a paper. Talanta 2015, 144, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tevis, I.D.; Oyola-Reynoso, S.; Newcomb, L.B.; Halbertsma-Black, J.; Thuo, M.; Bloch, J.-F. Melt-and-mold fabrication (MnM-Fab) of reconfigurable low-cost devices for use in resource-limited settings. Talanta 2015, 145, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.T.; Rolland, J.P.; Whitesides, G.M. “Paper machine” for molecular diagnostics. Anal. Chem. 2015, 87, 7595–7601. [Google Scholar] [CrossRef] [PubMed]
- Badu-Tawiah, A.K.; Lathwal, S.; Kaastrup, K.; Al-Sayah, M.H.; Christodouleas, D.C.; Smith, B.S.; Whitesides, G.M.; Sikes, H.D. Polymerization-based signal amplification for paper-based immunoassays. Lab Chip 2015, 15, 655–659. [Google Scholar] [CrossRef] [PubMed]
- Kumar Ashok, A.; Chunda-Liyoka, C.; Mantina, H.; Sambo, P.; Sinyangwe, S.; Kankasa, C.; Chintu, C.; Brugnara, C.; Stossel, T.P.; Whitesides, G.M.; et al. Evaluation of a density-based rapid diagnostic test for sickle cell disease in a clinical setting in Zambia. PLoS ONE 2014, 9, e114540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesides, G.M. A glimpse into the future of diagnostics. Clin Chem. 2013, 5, 589. [Google Scholar] [CrossRef] [PubMed]
- Bwambok, D.K.; Thuo, M.M.; Atkinson, M.B.J.; Mirica, K.A.; Shapiro, N.D.; Whitesides, G.M. Paramagnetic ionic liquids for measurements of density using magnetic levitation. Anal. Chem. 2013, 85, 8442–8447. [Google Scholar] [CrossRef] [PubMed]
- Alemnji, G.; Nkengasong, J.N.; Parekh, B.S. HIV testing in developing countries: What is required? Indian J. Med. Res. 2011, 134, 779–786. [Google Scholar] [PubMed]
- Ling, I.T.; Cooksley, S.; Bates, P.A.; Hempelmann, E.; Wilson, R.J.M. Antibodies to the glutamate-dehydrogenase of plasmodium-falciparum. Parasitology 1986, 92, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.W.; Phillips, S.T.; Whitesides, G.M.; Carrilho, E. Diagnostics for the developing world: Microfluidic paper-based analytical devices. Anal. Chem. 2010, 82, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.D.; Laksanasopin, T.; Cheung, Y.K.; Steinmiller, D.; Linder, V.; Parsa, H.; Wang, J.; Moore, H.; Rouse, R.; Umviligihozo, G.; et al. Microfluidics-based diagnostics of infectious diseases in the developing world. Nat. Med. 2011, 17, 1015–1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical biosensors: Towards point-of-care cancer diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Domingo, G.J.; Gerdes, J. Point-of-care diagnostics for global health. Ann. Rev. Biomed. Eng. 2008, 10, 107–144. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Myers, F.B.; Lee, L.P. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab Chip 2008, 8, 2015–2031. [Google Scholar] [CrossRef] [PubMed]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nature 2006, 442, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R. Bioactive paper provides a low-cost platform for diagnostics. Trac-Trends Anal. Chem. 2009, 28, 925–942. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007. [Google Scholar] [CrossRef]
- Miller, E.; Sikes, H.D. Addressing barriers to the development and adoption of rapid diagnostic test in global health. Nanobiomedicine 2015, 2, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Thiam, S.; Thior, M.; Faye, B.; Ndiop, M.; Diouf, M.L.; Diouf, M.B.; Diallo, I.; Fall, F.B.; Ndiaye, J.L.; Albertini, A.; et al. Major reduction in anti-malarial drug consumption in Senegal after nation-wide introduction of malaria rapid diagnostic tests. PLoS ONE 2011, 6, e18419. [Google Scholar] [CrossRef] [PubMed]
- Chandler, C.I.R.; Whitty, C.J.M.; Ansah, E.K. How can malaria rapid diagnostic tests achieve their potential? A qualitative study of a trial at health facilities in Ghana. Malar. J. 2010. [Google Scholar] [CrossRef] [PubMed]
- Skarbinski, J.; Ouma, P.O.; Causer, L.M.; Kariuki, S.K.; Barnwell, J.W.; Alaii, J.A.; de Oliveira, A.M.; Zurovac, D.; Larson, B.A.; Skarbinski, J.; et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether-lumefantrine in Kenya: A cluster randomized trial. Am. J. Trop. Med. Hyg. 2009, 80, 919–926. [Google Scholar] [PubMed]
- Uzochukwu, B.S.C.; Chiegboka, L.O.; Enwereuzo, C.; Nwosu, U.; Okorafor, D.; Onwujekwe, O.E.; Uguru, N.P.; Sibcudu, F.T.; Ezcokc, O.P. Examining appropriate diagnosis and treatment of malaria: Availability and use of rapid diagnostic tests and artemisinin-based combination therapy in public and private health facilities in south east Nigeria. BMC Public Health 2010, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, C.; Kyabayinze, D.J.; Kyalisiima, Z.; Nabakooza, J.; Bajabaite, M.; Counihan, H.; Tibenderana, J.K. Early experiences on the feasibility, acceptability, and use of malaria rapid diagnostic tests at peripheral health centres in Uganda-insights into some barriers and facilitators. Implement. Sci. 2012, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Pulcini, C.; Pauvif, L.; Paraponaris, A.; Verger, P.; Ventelou, B. Perceptions and attitudes of French general practitioners towards rapid antigen diagnostic tests in acute pharyngitis using a randomized case vignette study. J. Antimicrob. Chemother. 2012, 67, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.A.; Causer, L.; Metta, E.; Malila, A.; O’Reilly, T.; Abdulla, S.; Kachur, S.P.; Bloland, P.B. Dispensary level pilot implementation of rapid diagnostic tests: An evaluation of RDT acceptance and usage by providers and patients—Tanzania, 2005. Malar. J. 2008, 7, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baiden, F.; Webster, J.; Tivura, M.; Delimini, R.; Berko, Y.; Amenga-Etego, S.; Agyeman-Budu, A.; Karikari, A.B.; Bruce, J.; Owusu-Agyei, S.; et al. Accuracy of rapid tests for malaria and treatment outcomes for malaria and non-malaria cases among under-five children in rural Ghana. PLoS ONE 2012, 7, e34073. [Google Scholar] [CrossRef] [PubMed]
- Rogers, E.M. Diffusion of Innovations, 4th ed.; The Free Press: New York, NY, USA, 1995. [Google Scholar]
- Venkatesh, V. Determinants of perceived ease of use: Integrating control, intrinsic motivation, and emotion into the technology acceptance model. Inf. Syst. Res. 2000, 11, 342–365. [Google Scholar] [CrossRef]
- Gefen, D.; Karahanna, E.; Straub, D.W. Trust and TAM in online shopping: An integrated model. MIS Q. 2003, 27, 51–90. [Google Scholar]
- Chandra, A.; Calderon, T. Challenges and constraints to the diffusion of biometrics in information systems. Commun. ACM 2005, 48, 101–106. [Google Scholar] [CrossRef]
- Ring, P.S.; Vandeven, A.H. Structuring cooperative relationships between organizations. Strateg. Manag. J. 1992, 13, 483–498. [Google Scholar] [CrossRef]
- Bahmanziari, T.; Pearson, J.M.; Crosby, L. Is trust important in technology adoption? A policy capturing approach. J. Comput. Inf. Syst. 2003, 43, 46–54. [Google Scholar]
- Lippert, S.K.; Davis, M. A conceptual model integrating trust into planned change activities to enhance technology adoption behavior. J. Inf. Sci. 2006, 32, 432–448. [Google Scholar] [CrossRef]
- Sill, H.E.; Fisher, S.L.; Wasserman, M.E. Consumer reactions to potential intrusiveness and benefits of RFID. Int. J. Inf. Technol. Manag. 2007, 7, 76–97. [Google Scholar] [CrossRef]
- Nunnally, J.C. Psychometric Theory, 2nd ed.; McGraw-Hill: New York, NY, USA, 1978. [Google Scholar]
- Kossiakoff, A.; Sweet, W.N.; Seymour, S.J.; Biemer, S.M. Systems Engineering Principles and Practice, 2nd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mesmer, B.L.; Bloebaum, C.L. An end-user decision model with information representation for improved performance and robustness in complex system design. Res. Eng. Des. 2015, 26, 235–251. [Google Scholar] [CrossRef]
- Kwasa, B.J.; Bloebaum, C.L.; Mesmer, B.L.; Kannan, H.; Tibor, E. Value Impact of an Organization Structure in the Context of Value-Driven Design. In Proceedings of the 2015 AIAA 56th Structures, Structural Dynamics, and Materials Conference, Kissimmee, FL, USA, 5–9 January 2015. [Google Scholar]
- The World Health Organization. Global Update on the Health Sector Response to HIV; The World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Easterly, W. The White Man’s Burden: Why the West’s Efforts to Aid the Rest Have Done so Much Ill and So Little Good; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Rodney, W. How Europe Underdeveloped Africa; Bogle-L’Ouverture: London, UK, 1972. [Google Scholar]
- The World Health Organization. World Malaria Report 2014; The World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- The World Bank. Mind, Society and Behavior; World Development Report; The World Bank: Washington, DC, USA, 2015. [Google Scholar]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimani, F.W.; Mwangi, S.M.; Kwasa, B.J.; Kusow, A.M.; Ngugi, B.K.; Chen, J.; Liu, X.; Cademartiri, R.; Thuo, M.M. Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices. Micromachines 2017, 8, 317. https://doi.org/10.3390/mi8110317
Kimani FW, Mwangi SM, Kwasa BJ, Kusow AM, Ngugi BK, Chen J, Liu X, Cademartiri R, Thuo MM. Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices. Micromachines. 2017; 8(11):317. https://doi.org/10.3390/mi8110317
Chicago/Turabian StyleKimani, Faith W., Samuel M. Mwangi, Benjamin J. Kwasa, Abdi M. Kusow, Benjamin K. Ngugi, Jiahao Chen, Xinyu Liu, Rebecca Cademartiri, and Martin M. Thuo. 2017. "Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices" Micromachines 8, no. 11: 317. https://doi.org/10.3390/mi8110317
APA StyleKimani, F. W., Mwangi, S. M., Kwasa, B. J., Kusow, A. M., Ngugi, B. K., Chen, J., Liu, X., Cademartiri, R., & Thuo, M. M. (2017). Rethinking the Design of Low-Cost Point-of-Care Diagnostic Devices. Micromachines, 8(11), 317. https://doi.org/10.3390/mi8110317