A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Device Fabrication and Operating Principle
2.3. Conductive Anodic Reservoir
2.4. Air-Cathode
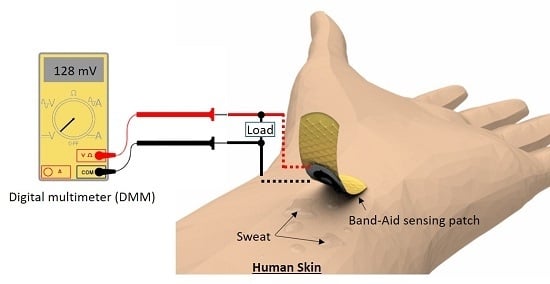
2.5. Measurement Setup
3. Results and Discussion
3.1. In Vitro Measurement with Artificial Sweat
3.2. In Vivo Measurement with Human Sweat
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dompsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.D.; Ma, X.; Maahs, D.M.; Trilk, J.L. Physical activity, sedentary behaviors, physical fitness, and their relation to health outcomes in youth with type 1 and type 2 diabetes: A review of the epidemiologic literature. J. Sport Health Sci. 2013, 2, 21–38. [Google Scholar] [CrossRef]
- Younk, L.M.; Mikeladze, M.; Tate, D.; Davis, S.N. Exercise-related hypoglycemia in diabetes mellitus. Expert Rev. Endocrinol. Metab. 2011, 6, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Sigal, R.J. Exercise strategies for hypoglycemia prevention in individuals with type 1 diabetes. Diabetes Spectr. 2015, 28, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Gallen, I.W. Hypoglycemia associated with exercise in people with type 1 diabetes. Diabet. Hypoglycemia 2014, 7, 3–10. [Google Scholar]
- Mahmoudi, Z.; Jensen, M.H.; Dencker, J.M.; Christensen, T.F.; Tarnow, L.; Christiansen, J.S.; Hejlesen, O. Accuracy evaluation of a new real-time continuous glucose monitoring algorithm in hypoglycemia. Diabetes Technol. Ther. 2014, 16, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Gifford, R. Continuous Glucose Monitoring: 40 years, What we’ve Learned and What’s Next. ChemPhysChem 2013, 14, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Witkowska Nery, E.; Kundys, M.; Jeleń, P.S.; Jönsson-Niedziółka, M. Electrochemical Glucose Sensing: Is There Still Room for Improvement? Anal. Chem. 2016, 88, 11271–11282. [Google Scholar] [CrossRef] [PubMed]
- Bandodkar, A.J.; Wang, J. Non-invasive wearable electrochemical sensors: A review. Trends Bio-Technol. 2014, 32, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.D.L. Sweat as a clinical sample: What is done and what should be done. Bioanalysis 2016, 8, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Makaram, P.; Owens, D.; Aceros, J. Trends in Nanomaterial-based Non-invasive Diabetes Sensing Technologies. Diagnostics 2014, 4, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Ainla, A.; Guder, F.; Christodouleas, D.C.; Fernandez-Abedul, M.T.; Whitesides, G.M. Integrating Electronics and Microfluidics on Paper. Adv. Mater. 2016, 28, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, M.M.; Campbell, V.E.; Rothemund, P.; Guder, F.; Christodouleas, D.C.; Bloch, J.F.; Whitesides, G.M. Electrically Activated Paper Actuators. Adv. Funct. Mater. 2016, 26, 2446–2453. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Zhao, F.; Xu, Z.; Dong, S. The direct electron transfer of glucose oxidase and glucose biosensor based on carbon nano-tube/chitosan matrix. Biosens. Bioelectron. 2005, 21, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, J.; Dong, S. Organically modified sol-gel/chitosan composite based glucose bio-sensor. Electroanalysis 2003, 15, 608–612. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent advances in electrochemical glucose biosensors: A review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Zhu, Z.; Garcia-Gancedo, L.; Flewitt, A.J.; Xie, H.; Moussy, F.; Milne, W.I. A critical review of glu-cose biosensors based on carbon nanomaterials: Carbon nanotubes and graphene. Sensors 2012, 12, 5996–6022. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Gao, Y.; Sun, J.; Gao, F. Mediatorless Glucose Biosensor and Direct Electron Transfer Type Glucose/Air Biofuel Cell Enabled with Carbon Nanodots. Anal. Chem. 2015, 87, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, S. An origami paper-based bacteria-powered battery. Nano Energy 2015, 15, 549–557. [Google Scholar] [CrossRef]
- Fraiwan, A.; Kwan, L.; Choi, S. A disposable power source in resource-limited environments: A paper-based biobattery generating electricity from wastewater. Biosens. Bioelectron. 2016, 85, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Nyein, H.Y.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z.; et al. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M. Recent Progress on the Development of Biofuel Cells for Self-Powered Electrochemical Biosensing and Logic Biosensing: A Review. Electroanalysis 2015, 27, 1786–1810. [Google Scholar] [CrossRef]
- Choi, S. Powering Point-of-care Diagnostic De-vices. Biotechnol. Adv. 2016, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Self-powered nanosensors and nanosystems. Adv. Mater. 2012, 24, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Fang, Y.; Wu, H.; Ahmad, M.; Luo, Z.; Li, Q.; Xie, J.; Yan, X.; Wu, L.; Wang, Z.L.; et al. Generating Electricity from Biofluid with a Nanowire-Based Biofuel Cell for Self-Powered Nanodevices. Adv. Mater. 2010, 22, 5388–5392. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D paper-based enzymatic fuel cell for self-powered, low-cost glucose monitoring. Biosens. Bioelectron. 2016, 79, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.T.; Lau, C.; Brozik, S.; Atanassov, P.; Banta, S. Engineering of glucose oxidase for direct electron transfer via site-specific gold nano-particle conjugation. JACS 2011, 133, 19262–19265. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Perez, T.; Hong, S.G.; Kim, J.; Ha, S. Entrap-ping cross-linked glucose oxidase aggregates within a graphitized mesoporous carbon net-work for enzymatic biofuel cells. Enzyme Microb. Technol. 2016, 90, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Choi, S. Cellular Flow in Paper-based Microfluidics. Sens. Actuators B 2016, 237, 1021–1026. [Google Scholar] [CrossRef]
- Fan, Y.; Sharbrough, E.; Liu, H. Quantification of the internal resistance distribution of microbial fuel cells. Environ. Sci. Technol. 2008, 42, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation between sweat glucose and blood glucose in subjects with diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kostov, Y.; Ge, K.; Rao, G.; Tolosa, L. Portable system for the detection of micromolar concentration of glucose. Meas. Sci. Technol. 2014, 25, 025701. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Ratterman, M.E.; Griffin, D.K.; Hou, L.; Kelly-Loughnance, N.; Naik, R.R.; Hagen, J.A.; Papautsky, I.; Heikenfeld, J.C. Adhesive RFID Sensor Patch for monitoring of sweat electrolytes. IEEE Trans. Biomed. Eng. 2015, 62, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J. Non-invasive analyte access and sensing through eccrine sweat: Challenges and outlook circa 2016. Electroanalysis 2016, 28, 1242–1249. [Google Scholar] [CrossRef]






| In Vivo Measurement | Blood | Sweat | |
|---|---|---|---|
| Glucose Level Measured from a Glucose Meter | Current at 10 kΩ | Glucose Level Estimation from the Calibration Curve | |
| Subject #1 | 0.96 mg/mL | 8.26 µA | 0.044 mg/mL |
| Subject #2 | 0.85 mg/mL | 8.10 µA | 0.021 mg/mL |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, E.; Mohammadifar, M.; Choi, S. A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia. Micromachines 2017, 8, 265. https://doi.org/10.3390/mi8090265
Cho E, Mohammadifar M, Choi S. A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia. Micromachines. 2017; 8(9):265. https://doi.org/10.3390/mi8090265
Chicago/Turabian StyleCho, Eunyoung, Maedeh Mohammadifar, and Seokheun Choi. 2017. "A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia" Micromachines 8, no. 9: 265. https://doi.org/10.3390/mi8090265
APA StyleCho, E., Mohammadifar, M., & Choi, S. (2017). A Single-Use, Self-Powered, Paper-Based Sensor Patch for Detection of Exercise-Induced Hypoglycemia. Micromachines, 8(9), 265. https://doi.org/10.3390/mi8090265