Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
Abstract
:1. Introduction
1.1. The Peritoneum Is Poorly Vascularized
1.2. Interstitial Fluid Pressure Is Increased in PM
2. Pharmacokinetic Aspects of Intraperitoneal Chemotherapy
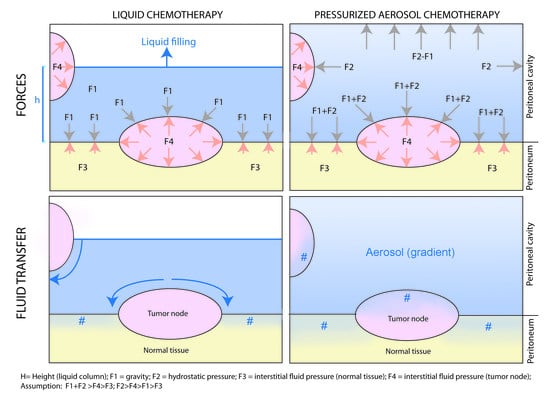
3. Physical Laws Governing Intraperitoneal Chemotherapy
4. Physical Interventions to Increase Drug Uptake
4.1. Increasing Pressure
4.2. Use of Aerosols
4.3. Adding Hyperthermia
5. Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
6. Preclinical Evidence of PIPAC
6.1. Effect of Hydrostatic Pressure
6.2. Homogeneity of Distribution
6.3. Tissue Concentration
6.4. Depth of Tissue Penetration
6.5. Local Effect vs. Systemic Uptake
7. Clinical Evidence on PIPAC in Chemoresistant PM
7.1. Local Efficacy
7.2. Local Toxicity
7.3. Systemic Toxicity
8. Limitations of PIPAC
9. Electrostatic Precipitation Pressurized Intraperitoneal Aerosol Chemotherapy (ePIPAC)
10. Hyperthermic Pressurized Intraperitoneal Aerosol Chemotherapy (hPIPAC)
11. In Silico Modelling
12. Conclusions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Arnold, D.; Taniguchi, H.; Pentheroudakis, G.; Yamazaki, K.; Xu, R.H.; Kim, T.W.; Ismail, F.; Tan, I.B.; Yeh, K.H.; et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018, 29, 44–70. [Google Scholar]
- Lambert, L.A. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J. Clin. 2015, 65, 284–298. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar]
- Kerscher, A.G.; Chua, T.C.; Gasser, M.; Maeder, U.; Kunzmann, V.; Isbert, C.; Germer, C.T.; Pelz, J.O. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer 2013, 108, 1432–1439. [Google Scholar] [CrossRef] [Green Version]
- Dahdaleh, F.S.; Turaga, K.K. Evolving Treatment Strategies and Outcomes in Advanced Gastric Cancer with Peritoneal Metastasis. Surg. Oncol. Clin. N. Am. 2018, 27, 519–537. [Google Scholar] [CrossRef]
- Jain, R.K. Barriers to drug delivery in solid tumors. Sci. Am. 1994, 271, 58–65. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Solass, W.; Horvath, P.; Struller, F.; Königsrainer, I.; Beckert, S.; Königsrainer, A.; Weinreich, F.J.; Schenk, M. Functional vascular anatomy of the peritoneum in health and disease. Pleura Peritoneum 2016, 1, 145–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamizi, S.; Freyer, G.; Bakrin, N.; Henin, E.; Mohtaram, A.; Le Saux, O.; Falandry, C. Subcutaneous trastuzumab: Development of a new formulation for treatment of HER2-positive early breast cancer. Onco. Targets Ther. 2013, 6, 89–94. [Google Scholar] [PubMed] [Green Version]
- Steuperaert, M.; Debbaut, C.; Carlier, C.; De Wever, O.; Descamps, B.; Vanhove, C.; Ceelen, W.; Segers, P. A 3D CFD model of the interstitial fluid pressure and drug distribution in heterogeneous tumor nodules during intraperitoneal chemotherapy. Drug Deliv. 2019, 26, 404–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Nathan, S.S.; Huvos, A.G.; Casas-Ganem, J.E.; Yang, R.; Linkov, I.; Sowers, R.; DiResta, G.R.; Gorlick, R.; Healey, J.H. Tumor interstitial fluid pressure may regulate angiogenic factors in osteosarcoma. J. Orthop. Res. 2008, 26, 1520–1525. [Google Scholar] [CrossRef]
- Milosevic, M.; Fyles, A.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R. Interstitial Fluid Pressure Predicts Survival in Patients with Cervix Cancer Independent of Clinical Prognostic Factors and Tumor Oxygen Measurements. Cancer Res. 2001, 61, 6400–6405. [Google Scholar]
- Rippe, B.; Haraldsson, B. Transport of macromolecules across microvascular walls: The two-pore theory. Physiol. Rev. 1994, 74, 163–219. [Google Scholar] [CrossRef]
- Li, L.; Li, W. Epithelial-mesenchymal transition in human cancer: Comprehensive reprogramming of metabolism, epigenetics, and differentiation. Pharmacol. Ther. 2015, 150, 33–46. [Google Scholar] [CrossRef]
- Wilson, R.B. Hypoxia, cytokines and stromal recruitment: Parallels between pathophysiology of encapsulating peritoneal sclerosis, endometriosis and peritoneal metastasis. Pleura Peritoneum 2018, 3, 20180103. [Google Scholar] [CrossRef]
- Lopez-Cabrera, M. Mesenchymal Conversion of Mesothelial Cells Is a Key Event in the Pathophysiology of the Peritoneum during Peritoneal Dialysis. Adv. Med. 2014, 2014, 473134. [Google Scholar] [CrossRef]
- De Bree, E.; Michelakis, D.; Stamatiou, D.; Romanos, J.; Zoras, O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum 2017, 2, 47–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sun, H.; Yakisich, J.S. Overcoming the blood-brain barrier for chemotherapy: Limitations, challenges and rising problems. Anticancer Agents Med. Chem. 2014, 14, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Loffler, M.W.; Schuster, H.; Zeck, A.; Quilitz, N.; Weinreich, J.; Tolios, A.; Haen, S.P.; Horvath, P.; Löb, S.; Rammensee, H.G.; et al. Pharmacodynamics of Oxaliplatin-Derived Platinum Compounds During Hyperthermic Intraperitoneal Chemotherapy (HIPEC): An Emerging Aspect Supporting the Rational Design of Treatment Protocols. Ann. Surg. Oncol. 2017, 24, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.M.; Sideris, L. Pharmacokinetics of heated intraoperative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis. Surg. Oncol. Clin. N. Am. 2003, 12, 755–769. [Google Scholar] [CrossRef]
- Ceelen, W. HIPEC with oxaliplatin for colorectal peritoneal metastasis: The end of the road? Eur. J. Surg. Oncol. 2019, 45, 400–402. [Google Scholar] [CrossRef]
- Flessner, M.F. Pharmacokinetic problems in peritoneal drug administration: An update after 20 years. Pleura Peritoneum 2016, 1, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Flessner, M.F. Small-solute transport across specific peritoneal tissue surfaces in the rat. J. Am. Soc. Nephrol. 1996, 7, 225–233. [Google Scholar]
- Solass, W.; Hetzel, A.; Nadiradze, G.; Sagynaliev, E.; Reymond, M.A. Description of a novel approach for intraperitoneal drug delivery and the related device. Surg. Endosc. 2012, 26, 1849–1855. [Google Scholar] [CrossRef]
- Shariati, M.; Zhang, H.; Van de Sande, L.; Descamps, B.; Vanhove, C.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. High Pressure Nebulization (PIPAC) Versus Injection for the Intraperitoneal Administration of mRNA Complexes. Pharm. Res. 2019, 36, 126. [Google Scholar] [CrossRef]
- Giger-Pabst, U.; Bucur, P.; Roger, S.; Falkenstein, T.A.; Tabchouri, N.; Le Pape, A.; Lerondel, S.; Demtröder, C.; Salamé, E.; Ouaissi, M. Comparison of Tissue and Blood Concentrations of Oxaliplatin Administrated by Different Modalities of Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2019, 26, 4445–4451. [Google Scholar] [CrossRef]
- Jacquet, P.; Stuart, O.A.; Chang, D.; Sugarbaker, P.H. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anticancer Drugs 1996, 7, 596–603. [Google Scholar] [CrossRef]
- Esquis, P.; Consolo, D.; Magnin, G.; Pointaire, P.; Moretto, P.; Ynsa, M.D.; Beltramo, J.L.; Drogoul, C.; Simonet, M.; Benoit, L.; et al. High intra-abdominal pressure enhances the penetration and antitumor effect of intraperitoneal cisplatin on experimental peritoneal carcinomatosis. Ann. Surg. 2006, 244, 106–112. [Google Scholar] [CrossRef]
- Facy, O.; Al Samman, S.; Magnin, G.; Ghiringhelli, F.; Ladoire, S.; Chauffert, B.; Rat, P.; Ortega-Deballon, P. High pressure enhances the effect of hyperthermia in intraperitoneal chemotherapy with oxaliplatin: An experimental study. Ann. Surg. 2012, 256, 1084–1088. [Google Scholar] [CrossRef]
- Facy, O.; Combier, C.; Poussier, M.; Magnin, G.; Ladoire, S.; Ghiringhelli, F.; Chauffert, B.; Rat, P.; Ortega-Deballon, P. High pressure does not counterbalance the advantages of open techniques over closed techniques during heated intraperitoneal chemotherapy with oxaliplatin. Surgery 2015, 157, 72–78. [Google Scholar] [CrossRef]
- Garofalo, A.; Valle, M.; Garcia, J.; Sugarbaker, P.H. Laparoscopic intraperitoneal hyperthermic chemotherapy for palliation of debilitating malignant ascites. Eur. J. Surg. Oncol. 2006, 32, 682–685. [Google Scholar] [CrossRef]
- Petrillo, M.; Zucchetti, M.; Cianci, S.; Morosi, L.; Ronsini, C.; Colombo, A.; D’Incalci, M.; Scambia, G.; Fagotti, A. Pharmacokinetics of cisplatin during open and minimally-invasive secondary cytoreductive surgery plus HIPEC in women with platinum-sensitive recurrent ovarian cancer: A prospective study. J. Gynecol. Oncol. 2019, 30, e59. [Google Scholar] [CrossRef]
- Kusamura, S.; Azmi, N.; Fumagalli, L.; Baratti, D.; Guaglio, M.; Cavalleri, A.; Garrone, G.; Battaglia, L.; Barretta, F.; Deraco, M. Phase II randomized study on tissue distribution and pharmacokinetics of cisplatin according to different levels of intra-abdominal pressure (IAP) during HIPEC (NCT02949791). Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Hinds, W.C. Aerosol Technology, 2nd ed.; Wiley—Interscience: New York, NY, USA, 1999. [Google Scholar]
- Grange, D.K.; Kratz, L.E.; Braverman, N.E.; Kelley, R.I. CHILD syndrome caused by deficiency of 3beta-hydroxysteroid-delta8, delta7-isomerase. Am. J. Med. Genet. 2000, 90, 328–335. [Google Scholar] [CrossRef]
- Solass, W.; Struller, F.; Horvath, P.; Königsrainer, A.; Sipos, B.; Weinreich, F.J. Morphology of the peritoneal cavity and pathophysiological consequences. Pleura Peritoneum 2016, 1, 193–201. [Google Scholar] [CrossRef]
- Labiris, N.R.; Dolovich, M.B. Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. Br. J. Clin. Pharmacol. 2003, 56, 588–599. [Google Scholar] [CrossRef]
- Kienle, G.S. Fever in Cancer Treatment: Coley’s Therapy and Epidemiologic Observations. Glob. Adv. Health Med. 2012, 1, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Calderwood, S.K. Autophagy, protein aggregation and hyperthermia: A mini-review. Int. J. Hyperth. 2011, 27, 409–414. [Google Scholar] [CrossRef] [Green Version]
- Stephens, A.D.; Belliveau, J.F.; Sugarbaker, P.H. Intraoperative hyperthermic lavage with cisplatin for peritoneal carcinomatosis and sarcomatosis. Cancer Treat Res. 1996, 81, 15–30. [Google Scholar]
- Leebmann, H.; Piso, P. Hyperthermic intraperitoneal chemotherapy. Chirurg 2019, 90, 593–604. [Google Scholar] [CrossRef]
- Eveno, C.; Pocard, M. Randomized controlled trials evaluating cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in prevention and therapy of peritoneal metastasis: A systematic review. Pleura Peritoneum 2016, 1, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Konigsrainer, A.; Rau, B. Cytoreductive Surgery (CRS) and Hyperthermic IntraPeritoneal Chemotherapy (HIPEC): Don’t throw the baby out with the bathwater. Pleura Peritoneum 2018, 3, 20180131. [Google Scholar] [CrossRef]
- Zeff, N. Role of laparoscopy in initial tumour staging in advanced epithelial ovarian cancer: A systematic review. Pleura Peritoneum 2018, 3, 20180106. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.K.; Jara, M.; Alberto, M.; Rau, B. The role of Pressurized IntraPeritoneal Aerosol Chemotherapy in the management of gastric cancer: A systematic review. Pleura Peritoneum 2019, 4, 20180127. [Google Scholar] [CrossRef]
- Glatz, T.; Horvath, P.; Lang, S.A.; Archid, R.; Nadiradze, G. Staging laparoscopy and Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) for peritoneal metastasis: Safe access to the abdomen. Pleura Peritoneum 2019, 4, 20190004. [Google Scholar] [CrossRef]
- Ramos, R.F.; Scalon, F.M.; Scalon, M.M.; Dias, D.I. Staging laparoscopy in gastric cancer to detect peritoneal metastases: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2016, 42, 1315–1321. [Google Scholar] [CrossRef]
- Park, Y.S.; Son, S.Y.; Oo, A.M.; Jung do, H.; Shin, D.J.; Ahn, S.H.; Park do, J.; Kim, H.H. Eleven-year experience with 3000 cases of laparoscopic gastric cancer surgery in a single institution: Analysis of postoperative morbidities and long-term oncologic outcomes. Surg. Endosc. 2016, 30, 3965–3975. [Google Scholar] [CrossRef]
- Pedziwiatr, M.; Małczak, P.; Pisarska, M.; Major, P.; Wysocki, M.; Stefura, T.; Budzyński, A. Minimally invasive versus open pancreatoduodenectomy-systematic review and meta-analysis. Langenbecks Arch. Surg. 2017, 402, 841–851. [Google Scholar] [CrossRef] [Green Version]
- Reymond, M.A.; Hu, B.; Garcia, A.; Reck, T.; Köckerling, F.; Hess, J.; Morel, P. Feasibility of therapeutic pneumoperitoneum in a large animal model using a microvaporisator. Surg. Endosc. 2000, 14, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Solass, W.; e Sempoux, C.; Detlefsen, S.; Carr, N.J.; Bibeau, F. Peritoneal sampling and histological assessment of therapeutic response in peritoneal metastasis: Proposal of the Peritoneal Regression Grading Score (PRGS). Pleura Peritoneum 2016, 1, 99–107. [Google Scholar] [CrossRef]
- Solass, W.; Sempoux, C.; Carr, N.J.; Bibeau, F.; Neureiter, D.; Jäger, T.; Di Caterino, T.; Brunel, C.; Klieser, E.; Fristrup, C.W.; et al. Reproducibility of the peritoneal regression grading score for assessment of response to therapy in peritoneal metastasis. Histopathology 2019, 74, 1014–1024. [Google Scholar] [CrossRef] [Green Version]
- Blanco, A.; Giger-Pabst, U.; Solass, W.; Zieren, J.; Reymond, M.A. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC). Ann. Surg. Oncol. 2013, 20, 2311–2316. [Google Scholar] [CrossRef] [Green Version]
- Solass, W.; Kerb, R.; Mürdter, T.; Giger-Pabst, U.; Strumberg, D.; Tempfer, C.; Zieren, J.; Schwab, M.; Reymond, M.A. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: First evidence for efficacy. Ann. Surg. Oncol. 2014, 21, 553–559. [Google Scholar] [CrossRef]
- Kim, G.; Tan, H.L.; Chen, E.; Teo, S.C.; Jang, C.J.M.; Ho, J.; Ang, Y.; Ngoi, N.Y.L.; Chee, C.E.; Lieske, B.; et al. Study protocol: Phase 1 dose escalating study of Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) with oxaliplatin in peritoneal metastasis. Pleura Peritoneum 2018, 3, 20180118. [Google Scholar] [CrossRef]
- Dumont, F.; Senellart, H.; Pein, F.; Campion, L.; Glehen, O.; Goere, D.; Pocard, M.; Thibaudeau, E. Phase I/II study of oxaliplatin dose escalation via a laparoscopic approach using pressurized aerosol intraperitoneal chemotherapy (PIPOX trial) for nonresectable peritoneal metastases of digestive cancers (stomach, small bowel and colorectal): Rationale and design. Pleura Peritoneum 2018, 3, 20180120. [Google Scholar]
- Van De Sande, L.; Graversen, M.; Hubner, M.; Pocard, M.; Reymond, M.; Vaira, M.; Cosyns, S.; Willaert, W.; Ceelen, W. Intraperitoneal aerosolization of albumin-stabilized paclitaxel nanoparticles (Abraxane) for peritoneal carcinomatosis—A phase I first-in-human study. Pleura Peritoneum 2018, 3, 20180112. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Winnekendonk, G.; Solass, W.; Horvat, R.; Giger-Pabst, U.; Zieren, J.; Rezniczek, G.A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: A phase 2 study. Gynecol. Oncol. 2015, 137, 223–228. [Google Scholar] [CrossRef]
- Khomyakov, V.; Ryabov, A.; Ivanov, A.; Bolotina, L.; Utkina, A.; Volchenko, N.; Kaprin, A. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and Doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 2016, 1, 159–166. [Google Scholar]
- Graversen, M.; Detlefsen, S.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Adjuvant Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in resected high-risk colon cancer patients—Study protocol for the PIPAC-OPC3 Trial. A prospective, controlled phase 2 Study. Pleura Peritoneum 2018, 3, 20180107. [Google Scholar] [CrossRef] [Green Version]
- Struller, F.; Horvath, P.; Solass, W.; Weinreich, F.J.; Strumberg, D.; Kokkalis, M.K.; Fischer, I.; Meisner, C.; Königsrainer, A.; Reymond, M.A. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: A phase II study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919846402. [Google Scholar] [CrossRef] [Green Version]
- Graversen, M.; Detlefsen, S.; Asmussen, J.; Mahdi, B.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Treatment of peritoneal carcinomatosis with Pressurized IntraPeritoneal Aerosol Chemotherapy—PIPAC-OPC2. Pleura Peritoneum 2018, 3, 20180108. [Google Scholar] [CrossRef]
- Rovers, K.P.; Lurvink, R.J.; Wassenaar, E.C.; Kootstra, T.J.; Scholten, H.J.; Tajzai, R.; Deenen, M.J.; Nederend, J.; Lahaye, M.J.; Huysentruyt, C.J.; et al. Repetitive electrostatic pressurised intraperitoneal aerosol chemotherapy (ePIPAC) with oxaliplatin as a palliative monotherapy for isolated unresectable colorectal peritoneal metastases: Protocol of a Dutch, multicentre, open-label, single-arm, phase II study (CRC-PIPAC). BMJ Open 2019, 9, e030408. [Google Scholar]
- Bakrin, N.; Tempfer, C.; Scambia, G.; De Simone, M.; Gabriel, B.; Grischke, E.M.; Rau, B. PIPAC-OV3: A multicenter, open-label, randomized, two-arm phase III trial of the effect on progression-free survival of cisplatin and doxorubicin as Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) vs. chemotherapy alone in patients with platinum-resistant recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer. Pleura Peritoneum 2018, 3, 20180114. [Google Scholar]
- Oliver Goetze, T.; Al-Batran, S.E.; Pabst, U.; Reymond, M.; Tempfer, C.; Bechstein, W.O.; Bankstahl, U.; Gockel, I.; Königsrainer, A.; Kraus, T.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in combination with standard of care chemotherapy in primarily untreated chemo naive upper gi-adenocarcinomas with peritoneal seeding—A phase II/III trial of the AIO/CAOGI/ACO. Pleura Peritoneum 2018, 3, 20180113. [Google Scholar] [CrossRef]
- Tempfer, C.B.; Giger-Pabst, U.; Seebacher, V.; Petersen, M.; Dogan, A.; Rezniczek, G.A. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol. Oncol. 2018, 150, 23–30. [Google Scholar] [CrossRef]
- Eveno, C.; Jouvin, I.; Pocard, M. PIPAC EstoK 01: Pressurized IntraPeritoneal Aerosol Chemotherapy with cisplatin and doxorubicin (PIPAC C/D) in gastric peritoneal metastasis: A randomized and multicenter phase II study. Pleura Peritoneum 2018, 3, 20180116. [Google Scholar] [CrossRef]
- Sgarbura, O.; Gourgou, S.; Tosi, D.; Bakrin, N.; Bouazza, N.; Delaine, S.; De Forges, H.; Pocard, M.; Quénet, F. MESOTIP: Phase II multicenter randomized trial evaluating the association of PIPAC and systemic chemotherapy vs. systemic chemotherapy alone as 1st-line treatment of malignant peritoneal mesothelioma. Pleura Peritoneum 2019, 4, 20190010. [Google Scholar] [CrossRef]
- Hubner, M. In search of evidence—PIPAC on the fast lane. Pleura Peritoneum 2018, 3, 20180119. [Google Scholar] [CrossRef] [Green Version]
- Seitenfus, R.; Kalil, A.N.; de Barros, E.D.; Galeano Zettler, C.; Dos Santos, G.O.; Glehen, O.; Cereser Junior, C.H.; Ferreira, P.R.W. Assessment of the aerosol distribution pattern of a single-port device for intraperitoneal administration of therapeutic substances. Surg. Endosc. 2019, 33, 3503–3510. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Giger-Pabst, U. Exploring the Spatial Drug Distribution Pattern of Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Ann. Surg. Oncol. 2016, 23, 1220–1224. [Google Scholar] [CrossRef]
- Kakchekeeva, T.; Demtröder, C.; Herath, N.I.; Griffiths, D.; Torkington, J.; Solaß, W.; Dutreix, M.; Reymond, M.A. In Vivo Feasibility of Electrostatic Precipitation as an Adjunct to Pressurized Intraperitoneal Aerosol Chemotherapy (ePIPAC). Ann. Surg. Oncol. 2016, 23, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Solass, W.; Herbette, A.; Schwarz, T.; Hetzel, A.; Sun, J.S.; Dutreix, M.; Reymond, M.A. Therapeutic approach of human peritoneal carcinomatosis with Dbait in combination with capnoperitoneum: Proof of concept. Surg. Endosc. 2012, 26, 847–852. [Google Scholar] [CrossRef] [Green Version]
- Shariati, M.; Willaert, W.; Ceelen, W.; De Smedt, S.C.; Remaut, K. Aerosolization of Nanotherapeutics as a Newly Emerging Treatment Regimen for Peritoneal Carcinomatosis. Cancers 2019, 11, 906. [Google Scholar] [CrossRef] [Green Version]
- Jung do, H.; Son, S.Y.; Oo, A.M.; Park, Y.S.; Shin, D.J.; Ahn, S.H.; Park do, J.; Kim, H.H. Feasibility of hyperthermic pressurized intraperitoneal aerosol chemotherapy in a porcine model. Surg. Endosc. 2016, 30, 4258–4264. [Google Scholar] [CrossRef]
- Sautkin, I.; Solass, W.; Weinreich, F.J.; Königsrainer, A.; Schenk, M.; Thiel, K.; Reymond, M.A. A real-time ex vivo model (eIBUB) for optimizing intraperitoneal drug delivery as an alternative to living animal models. Pleura Peritoneum 2019, 4, 20190017. [Google Scholar] [CrossRef]
- Tavernier, C.; Passot, G.; Vassal, O.; Allaouchiche, B.; Decullier, E.; Bakrin, N.; Alyami, M.; Davigo, A.; Bonnet, J.M.; Louzier, V.; et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) might increase the risk of anastomotic leakage compared to HIPEC: An experimental study. Surg. Endosc. 2019, 1–8, in press. [Google Scholar] [CrossRef]
- Weinreich, J.; Struller, F.; Sautkin, I.; Giuashvili, S.; Reymond, M.; Königsrainer, A.; Schott, T.C. Chemosensitivity of various peritoneal cancer cell lines to HIPEC and PIPAC: Comparison of an experimental duplex drug to standard drug regimens in vitro. Invest. New Drugs 2019, 37, 415–423. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Giger-Pabst, U.; Khosrawipour, T.; Pour, Y.H.; Diaz-Carballo, D.; Förster, E.; Böse-Ribeiro, H.; Adamietz, I.A.; Zieren, J.; Fakhrian, K.; et al. Effect of Irradiation on Tissue Penetration Depth of Doxorubicin after Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) in a Novel Ex-Vivo Model. J. Cancer 2016, 7, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Khosrawipour, V.; Khosrawipour, T.; Falkenstein, T.A.; Diaz-Carballo, D.; Förster, E.; Osma, A.; Adamietz, I.A.; Zieren, J.; Fakhrian, K. Evaluating the Effect of Micropump(c) Position, Internal Pressure and Doxorubicin Dosage on Efficacy of Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) in an Ex Vivo Model. Anticancer Res. 2016, 36, 4595–4600. [Google Scholar] [CrossRef] [Green Version]
- Khosrawipour, V.; Khosrawipour, T.; Kern, A.J.; Osma, A.; Kabakci, B.; Diaz-Carballo, D.; Förster, E.; Zieren, J.; Fakhrian, K. Distribution pattern and penetration depth of doxorubicin after pressurized intraperitoneal aerosol chemotherapy (PIPAC) in a postmortem swine model. J. Cancer Res. Clin. Oncol. 2016, 142, 2275–2280. [Google Scholar] [CrossRef]
- Eveno, C.; Haidara, A.; Ali, I.; Pimpie, C.; Mirshahi, M.; Pocard, M. Experimental pharmacokinetics evaluation of chemotherapy delivery by PIPAC for colon cancer: First evidence for efficacy. Pleura Peritoneum 2017, 2, 103–109. [Google Scholar] [CrossRef]
- Khosrawipour, V.; Diaz-Carballo, D.; Acikelli, A.H.; Khosrawipour, T.; Falkenstein, T.A.; Wu, D.; Zieren, J.; Giger-Pabst, U. Cytotoxic effect of different treatment parameters in pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the in vitro proliferation of human colonic cancer cells. World J. Surg. Oncol. 2017, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Schubert, J.; Khosrawipour, V.; Chaudhry, H.; Arafkas, M.; Knoefel, W.T.; Pigazzi, A.; Khosrawipour, T. Comparing the cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the proliferation of in vitro colon carcinoma cells following pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J. Surg. Oncol. 2019, 17, 93. [Google Scholar] [CrossRef]
- Mikolajczyk, A.; Khosrawipour, V.; Kulas, J.; Kocielek, K.; Migdal, P.; Arafkas, M.; Khosrawipour, T. Release of doxorubicin from its liposomal coating via high intensity ultrasound. Mol. Clin. Oncol. 2019, 11, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Robella, M.; Vaira, M.; Argenziano, M.; Spagnolo, R.; Cavalli, R.; Borsano, A.; Gentilli, S.; De Simone, M. Exploring the Use of Pegylated Liposomal Doxorubicin (Caelyx((R))) as Pressurized Intraperitoneal Aerosol Chemotherapy. Front. Pharmacol. 2019, 10, 669. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Doxorubicin (accessed on 19 December 2019).
- Sugarbaker, P.H.; Van der Speeten, K.; Stuart, O.A.; Chang, D. Impact of surgical and clinical factors on the pharmacology of intraperitoneal doxorubicin in 145 patients with peritoneal carcinomatosis. Eur. J. Surg. Oncol. 2011, 37, 719–726. [Google Scholar] [CrossRef]
- Nowacki, M.; Alyami, M.; Villeneuve, L.; Mercier, F.; Hubner, M.; Willaert, W.; Ceelen, W.; Reymond, M.; Pezet, D.; Arvieux, C.; et al. Multicenter comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: An international survey study. Eur. J. Surg. Oncol. 2018, 44, 991–996. [Google Scholar] [CrossRef]
- Saenz Medina, J.; Asuero de Lis, M.S.; Galindo Alvarez, J.; Villafruela Sanz, J.; Correa Gorospe, C.; Cuevas Sánchez, B.; Linares Quevedo, A.I.; Páez Borda, A.; Pascual Santos, J.; Marcén Letosa, R.; et al. Modification of the hemodynamic parameters and peripheral vascular flow in a porcine experimental of model of laparoscopic nephrectomy. Arch. Esp. Urol. 2007, 60, 501–518. [Google Scholar]
- Schilling, M.K.; Redaelli, C.; Krähenbühl, L.; Signer, C.; Büchler, M.W. Splanchnic microcirculatory changes during CO2 laparoscopy. J. Am. Coll. Surg. 1997, 184, 378–382. [Google Scholar]
- Alyami, M.; Hübner, M.; Grass, F.; Bakrin, N.; Villeneuve, L.; Laplace, N.; Passot, G.; Glehen, O.; Kepenekian, V. Pressurised intraperitoneal aerosol chemotherapy: Rationale, evidence, and potential indications. Lancet Oncol. 2019, 20, e368–e377. [Google Scholar] [CrossRef]
- Markman, M. Intraperitoneal antineoplastic drug delivery: Rationale and results. Lancet Oncol. 2003, 4, 277–283. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Pfeiffer, P.; Lundell, L.; Mortensen, M.B. Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): Report of two cases and literature survey. Clin. Exp. Metastasis 2018, 35, 103–108. [Google Scholar] [CrossRef]
- Tempfer, C.; Giger-Pabst, U.; Hilal, Z.; Dogan, A.; Rezniczek, G.A. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal carcinomatosis: Systematic review of clinical and experimental evidence with special emphasis on ovarian cancer. Arch. Gynecol. Obstet. 2018, 298, 243–257. [Google Scholar] [CrossRef]
- Gohler, D.; Khosrawipour, V.; Khosrawipour, T.; Diaz-Carballo, D.; Falkenstein, T.A.; Zieren, J.; Stintz, M.; Giger-Pabst, U. Technical description of the microinjection pump (MIP((R))) and granulometric characterization of the aerosol applied for pressurized intraperitoneal aerosol chemotherapy (PIPAC). Surg. Endosc. 2017, 31, 1778–1784. [Google Scholar] [CrossRef]
- Bellendorf, A.; Khosrawipour, V.; Khosrawipour, T.; Siebigteroth, S.; Cohnen, J.; Diaz-Carballo, D.; Bockisch, A.; Zieren, J.; Giger-Pabst, U. Scintigraphic peritoneography reveals a non-uniform (99m)Tc-Pertechnetat aerosol distribution pattern for Pressurized Intra-Peritoneal Aerosol Chemotherapy (PIPAC) in a swine model. Surg. Endosc. 2018, 32, 166–174. [Google Scholar]
- Khosrawipour, V.; Mikolajczyk, A.; Schubert, J.; Khosrawipour. Pressurized Intra-peritoneal Aerosol Chemotherapy (PIPAC) via Endoscopical Microcatheter System. Anticancer Res. 2018, 38, 3447–3452. [Google Scholar] [CrossRef]
- Levin, E.A.; Ceelen, W.P. Intraperitoneal Cancer Therapy: Principles and Practice; CRC Press (Taylor and Francis): Abington, MA, USA, 2016; pp. 389–402. [Google Scholar]
- Ansell, J.; Warren, N.; Wall, P.; Cocks, K.; Goddard, S.; Whiston, R.; Stechman, M.; Scott-Coombes, D.; Torkington, J. Electrostatic precipitation is a novel way of maintaining visual field clarity during laparoscopic surgery: A prospective double-blind randomized controlled pilot study. Surg. Endosc. 2014, 28, 2057–2065. [Google Scholar]
- Reymond, M.; Demtroeder, C.; Solass, W.; Winnekendonk, G.; Tempfer, C. Electrostatic precipitation Pressurized IntraPeritoneal Aerosol Chemotherapy (ePIPAC): First in-human application. Pleura Peritoneum 2016, 1, 109–116. [Google Scholar] [CrossRef]
- Willaert, W.; Van de Sande, L.; Van Daele, E.; Van De Putte, D.; Van Nieuwenhove, Y.; Pattyn, P.; Ceelen, W. Safety and preliminary efficacy of electrostatic precipitation during pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable carcinomatosis. Eur. J. Surg. Oncol. 2019, 45, 2302–2309. [Google Scholar] [CrossRef]
- Graversen, M.; Detlefsen, S.; Ellebaek, S.B.; Fristrup, C.; Pfeiffer, P.; Mortensen, M.B. Pressurized IntraPeritoneal Aerosol Chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumor response in peritoneal metastasis is insufficient. Eur. J. Surg. Oncol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Steuperaert, M.; Debbaut, C.; Segers, P.; Ceelen, W. Modelling drug transport during intraperitoneal chemotherapy. Pleura Peritoneum 2017, 2, 73–83. [Google Scholar] [CrossRef] [Green Version]
- D’Esposito, A.; Sweeney, P.W.; Ali, M.; Saleh, M.; Ramasawmy, R.; Roberts, T.A.; Agliardi, G.; Desjardins, A.; Lythgoe, M.F.; Pedley, R.B.; et al. Computational fluid dynamics with imaging of cleared tissue and of in vivo perfusion predicts drug uptake and treatment responses in tumours. Nat. Biomed. Eng. 2018, 2, 773–787. [Google Scholar] [CrossRef] [Green Version]
- Rahimi-Gorji, M.B.S.; Debbaut, C.; Descamps, D.; Pullens, P.; Segers, P.; Sourbron, W.; Ceelen, W. Validation of a computational fluid dynamics model of drug delivery and interstitiaö fluid pressure in ovarian cancer xenografts. In Proceedings of the 5th ISSPP Workshop on Basic Science, Paris, France, 27 September 2019. [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadiradze, G.; Horvath, P.; Sautkin, Y.; Archid, R.; Weinreich, F.-J.; Königsrainer, A.; Reymond, M.A. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers 2020, 12, 34. https://doi.org/10.3390/cancers12010034
Nadiradze G, Horvath P, Sautkin Y, Archid R, Weinreich F-J, Königsrainer A, Reymond MA. Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers. 2020; 12(1):34. https://doi.org/10.3390/cancers12010034
Chicago/Turabian StyleNadiradze, Giorgi, Philipp Horvath, Yaroslav Sautkin, Rami Archid, Frank-Jürgen Weinreich, Alfred Königsrainer, and Marc A. Reymond. 2020. "Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)" Cancers 12, no. 1: 34. https://doi.org/10.3390/cancers12010034
APA StyleNadiradze, G., Horvath, P., Sautkin, Y., Archid, R., Weinreich, F. -J., Königsrainer, A., & Reymond, M. A. (2020). Overcoming Drug Resistance by Taking Advantage of Physical Principles: Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC). Cancers, 12(1), 34. https://doi.org/10.3390/cancers12010034