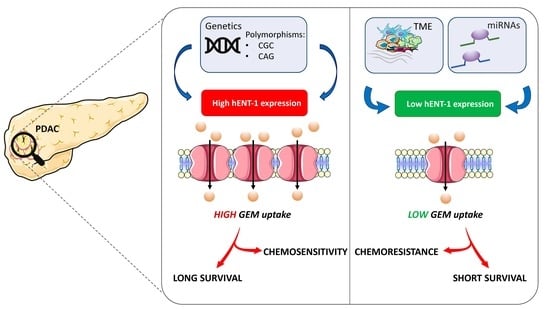
“Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pancreatic Ductal Adenocarcinoma (PDAC)
3. Nucleoside Transporters Involved in Gemcitabine Uptake
4. Role of hENT-1 in Gemcitabine Activity as Potential Predictive Biomarker
5. Evaluation of the Study “hENT-1 Status in PDAC Patients—Are We Ready Yet?”
6. Factors Involved in hENT1 Regulation and Gemcitabine Activity
6.1. Genetics: Mutations and Polymorphisms
6.2. Epigenetics and microRNAs
6.2.1. Epigenetics
6.2.2. microRNAs
6.3. Tumour Microenvironment
6.3.1. Hypoxia
6.3.2. Mechanobiology
7. Discussion
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molina-Arcas, M.; Trigueros-Motos, L.; Casado, F.J.; Pastor-Anglada, M. Physiological and Pharmacological Roles of Nucleoside Transporter Proteins. Nucleosides Nucleotides Nucleic Acids 2008, 27, 769–778. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef]
- Raffenne, J.; Nicolle, R.; Puleo, F.; Le Corre, D.; Boyez, C.; Marechal, R.; Emile, J.F.; Demetter, P.; Bardier, A.; Laurent-Puig, P.; et al. hENT1 Testing in Pancreatic Ductal Adenocarcinoma: Are We Ready? A Multimodal Evaluation of hENT1 Status. Cancers 2019, 11, 1808. [Google Scholar] [CrossRef] [Green Version]
- El Hassouni, B.; Li Petri, G.; Liu, D.S.K.; Cascioferro, S.; Parrino, B.; Hassan, W.; Diana, P.; Ali, A.; Frampton, A.E.; Giovannetti, E. Pharmacogenetics of treatments for pancreatic cancer. Expert Opin. Drug Metab. Toxicol. 2019, 15, 437–447. [Google Scholar] [CrossRef]
- Poplin, E.; Wasan, H.; Rolfe, L.; Raponi, M.; Ikdahl, T.; Bondarenko, I.; Davidenko, I.; Bondar, V.; Garin, A.; Boeck, S.; et al. Randomized, Multicenter, Phase II Study of CO-101 Versus Gemcitabine in Patients With Metastatic Pancreatic Ductal Adenocarcinoma: Including a Prospective Evaluation of the Role of hENT1 in Gemcitabine or CO-101 Sensitivity. J. Clin. Oncol. 2013, 31, 4453–4461. [Google Scholar] [CrossRef]
- Blagden, S.P.; Rizzuto, I.; Suppiah, P.; O’Shea, D.; Patel, M.; Spiers, L.; Sukumaran, A.; Bharwani, N.; Rockall, A.; Gabra, H.; et al. Anti-tumour activity of a first-in-class agent NUC-1031 in patients with advanced cancer: Results of a phase I study. Br. J. Cancer 2018, 119, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J. Clin. 2020. [Google Scholar] [CrossRef]
- Collisson, E.A.; Bailey, P.; Chang, D.K.; Biankin, A.V. Molecular subtypes of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 207–220. [Google Scholar] [CrossRef]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef]
- Giovannetti, E.; van der Borden, C.L.; Frampton, A.E.; Ali, A.; Firuzi, O.; Peters, G.J. Never let it go: Stopping key mechanisms underlying metastasis to fight pancreatic cancer. Semin. Cancer Biol. 2017, 44, 43–59. [Google Scholar] [CrossRef]
- Adamska, A.; Domenichini, A.; Falasca, M. Pancreatic Ductal Adenocarcinoma: Current and Evolving Therapies. Int. J. Mol. Sci. 2017, 18, 1338. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Caparello, C.; Meijer, L.L.; Garajova, I.; Falcone, A.; Le Large, T.Y.; Funel, N.; Kazemier, G.; Peters, G.J.; Vasile, E.; Giovannetti, E. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J. Gastroenterol. 2016, 22, 6987–7005. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valle, S.; Martin-Hijano, L.; Alcalá, S.; Alonso-Nocelo, M.; Sainz, B., Jr. The Ever-Evolving Concept of the Cancer Stem Cell in Pancreatic Cancer. Cancers 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Zeng, S.; Pöttler, M.; Lan, B.; Grützmann, R.; Pilarsky, C.; Yang, H. Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2019, 20, 4504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principe, D.R.; DeCant, B.; Mascarinas, E.; Wayne, E.A.; Diaz, A.M.; Akagi, N.; Hwang, R.; Pasche, B.; Dawson, D.W.; Fang, D.; et al. TGF Signaling in the Pancreatic Tumor Microenvironment Promotes Fibrosis and Immune Evasion to Facilitate Tumorigenesis. Cancer Res. 2016, 76, 2525–2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Young, J.D.; Yao, S.Y.M.; Sun, L.; Cass, C.E.; Baldwin, S.A. Human equilibrative nucleoside transporter (ENT) family of nucleoside and nucleobase transporter proteins. Xenobiotica 2008, 38, 995–1021. [Google Scholar] [CrossRef]
- King, A.E.; Ackley, M.A.; Cass, C.E.; Young, J.D.; Baldwin, S.A. Nucleoside transporters: From scavengers to novel therapeutic targets. Trends Pharmacol. Sci. 2006, 27, 416–425. [Google Scholar] [CrossRef]
- Govindarajan, R.; Bakken, A.H.; Hudkins, K.L.; Lai, Y.; Casado, F.J.; Pastor-Anglada, M.; Tse, C.-M.; Hayashi, J.; Unadkat, J.D. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1809–R1822. [Google Scholar] [CrossRef] [Green Version]
- Pastor-Anglada, M.; Urtasun, N.; Perez-Torras, S. Intestinal Nucleoside Transporters: Function, Expression, and Regulation. Compr. Physiol. 2018, 8, 1003–1017. [Google Scholar] [CrossRef]
- Rauchwerger, D.R.; Firby, P.S.; Hedley, D.W.; Moore, M.J. Equilibrative-sensitive nucleoside transporter and its role in gemcitabine sensitivity. Cancer Res. 2000, 60, 6075–6079. [Google Scholar]
- Zhou, M.; Duan, H.; Engel, K.; Wang, J. Adenosine transport by plasma membrane monoamine transporter: Reinvestigation and comparison with organic cations. Drug Metab. Dispos. 2010, 38, 1798–1805. [Google Scholar] [CrossRef] [Green Version]
- Boswell-Casteel, R.C.; Hays, F.A. Equilibrative nucleoside transporters—A review. Nucleosides Nucleotides Nucleic Acids 2017, 36, 7–30. [Google Scholar] [CrossRef]
- Damaraju, V.L.; Damaraju, S.; Young, J.D.; Baldwin, S.A.; Mackey, J.; Sawyer, M.B.; Cass, C.E. Nucleoside anticancer drugs: The role of nucleoside transporters in resistance to cancer chemotherapy. Oncogene 2003, 22, 7524–7536. [Google Scholar] [CrossRef] [Green Version]
- Balboni, B.; El Hassouni, B.; Honeywell, R.J.; Sarkisjan, D.; Giovannetti, E.; Poore, J.; Heaton, C.; Peterson, C.; Benaim, E.; Lee, Y.B.; et al. RX-3117 (fluorocyclopentenyl cytosine): A novel specific antimetabolite for selective cancer treatment. Expert Opin. Investig. Drugs. 2019, 28, 311–322. [Google Scholar] [CrossRef]
- Wei, R.; Gust, S.L.; Tandio, D.; Maheux, A.; Nguyen, K.H.; Wang, J.; Bourque, S.; Plane, F.; Hammond, J.R. Deletion of murine slc29a4 modifies vascular responses to adenosine and 5-hydroxytryptamine in a sexually dimorphic manner. Physiol. Rep. 2020, 8. [Google Scholar] [CrossRef] [Green Version]
- Wright, N.J.; Lee, S.-Y. Structures of human ENT1 in complex with adenosine reuptake inhibitors. Nat. Struct. Mol. Biol. 2019, 26, 599–606. [Google Scholar] [CrossRef]
- Elnaggar, M.; Giovannetti, E.; Peters, G.J. Molecular Targets of Gemcitabine Action: Rationale for Development of Novel Drugs and Drug Combinations. Curr. Pharm. Des. 2012, 18, 2811–2829. [Google Scholar] [CrossRef] [PubMed]
- Cass, C.E.; Young, J.D.; Baldwin, S.A.; Cabrita, M.A.; Graham, K.A.; Griffiths, M.; Jennings, L.L.; Mackey, J.R.; Ng, A.M.L.; Ritzel, M.W.L.; et al. Nucleoside transporters of mammalian cells. Pharm. Biotechnol. 1999, 12, 313–352. [Google Scholar] [CrossRef]
- GEPIA. Available online: http://gepia.cancer-pku.cn/detail.php?gene=SLC29A1 (accessed on 28 October 2020).
- Griffiths, M.; Beaumont, N.; Yao, S.Y.M.; Sundaram, M.; Boumah, C.E.; Davies, A.; Kwong, F.Y.P.; Coe, I.; Cass, C.E.; Young, J.D.; et al. Cloning of a human nucleoside transporter implicated in the Cellular uptake of adenosine and chemotherapeutic drugs. Nat. Med. 1997, 3, 89–93. [Google Scholar] [CrossRef]
- Mackey, J.R.; Mani, R.S.; Selner, M.; Mowles, D.; Young, J.D.; Belt, J.A.; Crawford, C.R.; Cass, C.E. Functional nucleoside transporters are required for gemcitabine influx and manifestation of toxicity in cancer cell lines. Cancer Res. 1998, 58, 4349–4357. [Google Scholar] [PubMed]
- de Sousa Cavalcante, L.; Monteiro, G. Gemcitabine: Metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur. J. Pharmacol. 2014, 741, 8–16. [Google Scholar] [CrossRef]
- Achiwa, H.; Oguri, T.; Sato, S.; Maeda, H.; Niimi, T.; Ueda, R. Determinants of sensitivity and resistance to gemcitabine: The roles of human equilibrative nucleoside transporter 1 and deoxycytidine kinase in non-small cell lung cancer. Cancer Sci. 2004, 95, 753–757. [Google Scholar] [CrossRef] [Green Version]
- Mey, V.; Giovannetti, E.; Braud, F.D.; Nannizzi, S.; Curigliano, G.; Verweij, F.; Cobelli, O.D.; Pece, S.; Tacca, M.D.; Danesi, R. In vitro synergistic cytotoxicity of gemcitabine and pemetrexed and pharmacogenetic evaluation of response to gemcitabine in bladder cancer patients. Br. J Cancer 2006, 95, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Damaraju, V.L.; Scriver, T.; Mowles, D.; Kuzma, M.; Ryan, A.J.; Cass, C.E.; Sawyer, M.B. Erlotinib, Gefitinib, and Vandetanib Inhibit Human Nucleoside Transporters and Protect Cancer Cells from Gemcitabine Cytotoxicity. Clin. Cancer Res. 2014, 20, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Hubeek, I.; Giovannetti, E.; Broekhuizen, A.J.F.; Pastor-Anglada, M.; Kaspers, G.J.L.; Peters, G.J. Immunocytochemical Detection of hENT1 and hCNT1 in normal tissues, lung cancer cell lines, and NSCLC patient samples. Nucleosides Nucleotides Nucleic Acids 2008, 27, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.; Sangha, R.; Glubrecht, D.; Dabbagh, L.; Young, J.D.; Dumontet, C.; Cass, C.; Lai, R.; Mackey, J.R. The Absence of Human Equilibrative Nucleoside Transporter 1 Is Associated with Reduced Survival in Patients With Gemcitabine-Treated Pancreas Adenocarcinoma. Clin. Cancer Res. 2004, 10, 6956–6961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giovannetti, E.; Del Tacca, M.; Mey, V.; Funel, N.; Nannizzi, S.; Ricci, S.; Orlandini, C.; Boggi, U.; Campani, D.; Del Chiaro, M.; et al. Transcription Analysis of Human Equilibrative Nucleoside Transporter-1 Predicts Survival in Pancreas Cancer Patients Treated with Gemcitabine. Cancer Res. 2006, 66, 3928–3935. [Google Scholar] [CrossRef] [Green Version]
- Farrell, J.J.; Elsaleh, H.; Garcia, M.; Lai, R.; Ammar, A.; Regine, W.F.; Abrams, R.; Benson, A.B.; Macdonald, J.; Cass, C.E.; et al. Human Equilibrative Nucleoside Transporter 1 Levels Predict Response to Gemcitabine in Patients With Pancreatic Cancer. Gastroenterology 2009, 136, 187–195. [Google Scholar] [CrossRef]
- Fujita, H.; Ohuchida, K.; Mizumoto, K.; Itaba, S.; Ito, T.; Nakata, K.; Yu, J.; Kayashima, T.; Souzaki, R.; Tajiri, T.; et al. Gene Expression Levels as Predictive Markers of Outcome in Pancreatic Cancer after Gemcitabine-Based Adjuvant Chemotherapy. Neoplasia 2010, 12, 807–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morinaga, S.; Nakamura, Y.; Watanabe, T.; Mikayama, H.; Tamagawa, H.; Yamamoto, N.; Shiozawa, M.; Akaike, M.; Ohkawa, S.; Kameda, Y.; et al. Immunohistochemical Analysis of Human Equilibrative Nucleoside Transporter-1 (hENT1) Predicts Survival in Resected Pancreatic Cancer Patients Treated with Adjuvant Gemcitabine Monotherapy. Ann. Surg. Oncol. 2012, 19, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Maréchal, R.; Bachet, J.; Mackey, J.R.; Dalban, C.; Demetter, P.; Graham, K.; Couvelard, A.; Svrcek, M.; Bardier–Dupas, A.; Hammel, P.; et al. Levels of Gemcitabine Transport and Metabolism Proteins Predict Survival Times of Patients Treated with Gemcitabine for Pancreatic Adenocarcinoma. Gastroenterology 2012, 143, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Hamada, T.; Kishiwada, M.; Ohsawa, I.; Mizuno, S.; Usui, M.; Sakurai, H.; Tabata, M.; Ii, N.; Inoue, H.; et al. Human equilibrative nucleoside transporter 1 expression is a strong independent prognostic factor in UICC T3-T4 pancreatic cancer patients treated with preoperative gemcitabine-based chemoradiotherapy. J. Hepatobiliary Pancreat. Sci. 2012, 19, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Sueda, T. Combined analysis of intratumoral human equilibrative nucleoside transporter 1 (hENT1) and ribonucleotide reductase regulatory subunit M1 (RRM1) expression is a powerful predictor of survival in patients with pancreatic carcinoma treated with adjuvant gemcitabine-based chemotherapy after operative resection. Surgery 2013, 153, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Nordh, S.; Ansari, D.; Andersson, R. hENT1 expression is predictive of gemcitabine outcome in pancreatic cancer: A systematic review. World J. Gastroenterol. 2014, 20, 8482–8490. [Google Scholar] [CrossRef]
- Greenhalf, W.; Ghaneh, P.; Neoptolemos, J.P.; Palmer, D.H.; Cox, T.F.; Lamb, R.F.; Garner, E.; Campbell, F.; Mackey, J.R.; Costello, E.; et al. Pancreatic Cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef]
- Bird, N.T.E.; Elmasry, M.; Jones, R.; Psarelli, E.; Dodd, J.; Malik, H.; Greenhalf, W.; Kitteringham, N.; Ghaneh, P.; Neoptolemos, J.P.; et al. Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br. J. Surg. 2017, 104, 328–336. [Google Scholar] [CrossRef]
- Yamada, R.; Mizuno, S.; Uchida, K.; Yoneda, M.; Kanayama, K.; Inoue, H.; Murata, Y.; Kuriyama, N.; Kishiwada, M.; Usui, M.; et al. Human Equilibrative Nucleoside Transporter 1 Expression in Endoscopic Ultrasonography-Guided Fine-Needle Aspiration Biopsy Samples Is a Strong Predictor of Clinical Response and Survival in the Patients With Pancreatic Ductal Adenocarcinoma Undergoing Gemcitabine-Based Chemoradiotherapy. Pancreas 2016, 45, 761–771. [Google Scholar] [CrossRef] [Green Version]
- Brandi, G.; Deserti, M.; Vasuri, F.; Farioli, A.; Degiovanni, A.; Palloni, A.; Frega, G.; Barbera, M.A.; Lorenzo, S.; Garajova, I.; et al. Membrane Localization of Human Equilibrative Nucleoside Transporter 1 in Tumor Cells May Predict Response to Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients. Oncologist 2016, 21, 600–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, L.L.; Puik, J.R.; Peters, G.J.; Kazemier, G.; Giovannetti, E. hENT-1 Expression and Localization Predict Outcome After Adjuvant Gemcitabine in Resected Cholangiocarcinoma Patients. Oncologist 2016, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavolari, S.; Deserti, M.; Vasuri, F.; Curti, S.; Palloni, A.; Pinna, A.D.; Cescon, M.; Frega, G.; De Lorenzo, S.; Barbera, M.A.; et al. Membrane human equilibrative nucleoside transporter 1 is associated with a high proliferation rate and worse survival in resected intrahepatic cholangiocarcinoma patients not receiving adjuvant treatments. Eur. J. Cancer. 2019, 106, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Kawada, N.; Uehara, H.; Katayama, K.; Nakamura, S.; Takahashi, H.; Ohigashi, H.; Ishikawa, O.; Nagata, S.; Tomita, Y. Human equilibrative nucleoside transporter 1 level does not predict prognosis in pancreatic cancer patients treated with neoadjuvant chemoradiation including gemcitabine. J. Hepatobiliary Pancreat. Sci. 2012, 19, 717–722. [Google Scholar] [CrossRef]
- Sinn, M.; Riess, H.; Sinn, B.V.; Stieler, J.M.; Pelzer, U.; Striefler, J.K.; Oettle, H.; Bahra, M.; Denkert, C.; Bläker, H.; et al. Human equilibrative nucleoside transporter 1 expression analysed by the clone SP 120 rabbit antibody is not predictive in patients with pancreatic cancer treated with adjuvant gemcitabine—Results from the CONKO-001 trial. Eur. J. Cancer 2015, 51, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Ormanns, S.; Heinemann, V.; Raponi, M.; Isaacson, J.; Laubender, R.P.; Haas, M.; Kruger, S.; Kleespies, A.; Mann, E.; Bartosiewicz, M.; et al. Human equilibrative nucleoside transporter 1 is not predictive for gemcitabine efficacy in advanced pancreatic cancer: Translational results from the AIO-PK0104 phase III study with the clone SP120 rabbit antibody. Eur. J. Cancer 2014, 50, 1891–1899. [Google Scholar] [CrossRef]
- Svrcek, M.; Cros, J.; Maréchal, R.; Bachet, J.-B.; Fléjou, J.-F.; Demetter, P. Human equilibrative nucleoside transporter 1 testing in pancreatic ductal adenocarcinoma: A comparison between murine and rabbit antibodies. Histopathology 2015, 66, 457–462. [Google Scholar] [CrossRef]
- Kalloger, S.E.; Riazy, M.; Tessier-Cloutier, B.; Karasinska, J.M.; Gao, D.; Peixoto, R.D.; Samimi, S.; Chow, C.; Wong, H.-L.; Mackey, J.R.; et al. A predictive analysis of the SP120 and 10D7G2 antibodies for human equilibrative nucleoside transporter 1 (hENT1) in pancreatic ductal adenocarcinoma treated with adjuvant gemcitabine. J. Pathol. Clin. Res. 2017, 3, 179–190. [Google Scholar] [CrossRef]
- Funel, N.; Giovannetti, E.; Pollina, L.E.; del Chiaro, M.; Mosca, F.; Boggi, U.; Campani, D. Critical role of laser microdissection for genetic, epigenetic and proteomic analyses in pancreatic cancer. Expert Rev. Mol. Diagn. 2011, 11, 695–701. [Google Scholar] [CrossRef]
- Le Large, T.Y.S.; Mantini, G.; Meijer, L.L.; Pham, T.V.; Funel, N.; van Grieken, N.C.T.; Kok, B.; Knol, J.; van Laarhoven, H.W.M.; Piersma, S.R.; et al. Microdissected pancreatic cancer proteomes reveal tumor heterogeneity and therapeutic targets. JCI Insight 2020, 5, e138290. [Google Scholar] [CrossRef] [PubMed]
- Jiraskova, L.; Ryska, A.; Duintjer Tebbens, E.J.; Hornychova, H.; Cecka, F.; Staud, F.; Cerveny, L. Are ENT1/ENT1, NOTCH3, and miR-21 Reliable Prognostic Biomarkers in Patients with Resected Pancreatic Adenocarcinoma Treated with Adjuvant Gemcitabine Monotherapy? Cancers 2019, 11, 1621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Shen, J.; Lu, Y.; Lin, K.; Wang, H.; Li, Y.; Chang, P.; Walker, M.G.; Li, D. RNA sequencing analyses reveal novel differentially expressed genes and pathways in pancreatic cancer. Oncotarget 2017, 8, 42537–42547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Schetter, A.; He, P.; Funamizu, N.; Gaedcke, J.; Ghadimi, B.M.; Ried, T.; Hassan, R.; Yfantis, H.G.; Lee, D.H.; et al. DPEP1 inhibits tumor cell invasiveness, enhances chemosensitivity and predicts clinical outcome in pancreatic ductal adenocarcinoma. PLoS ONE 2012, 7, e31507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Wang, Q.; Wang, D.; Li, J.; Yang, J.; Li, H.; Wang, X.; Jin, X.; Jing, R.; Yang, J.-H.; et al. Label-Free Quantitative Proteomics Unravels Carboxypeptidases as the Novel Biomarker in Pancreatic Ductal Adenocarcinoma. Transl. Oncol. 2018, 11, 691–699. [Google Scholar] [CrossRef]
- Mantini, G.; Vallés, A.M.; Le Large, T.Y.S.; Capula, M.; Funel, N.; Pham, T.V.; Piersma, S.R.; Kazemier, G.; Bijlsma, M.F.; Giovannetti, E.; et al. Co-expression analysis of pancreatic cancer proteome reveals biology and prognostic biomarkers. Cell Oncol. 2020. [Google Scholar] [CrossRef]
- Jaramillo, A.C.; Hubeek, I.; Broekhuizen, R.; Pastor-Anglada, M.; Kaspers, G.J.L.; Jansen, G.; Cloos, J.; Peters, G.J. Expression of the nucleoside transporters hENT1 (SLC29) and hCNT1 (SLC28) in pediatric acute myeloid leukemia. Nucleosides Nucleotides Nucleic Acids 2020, 1–10. [Google Scholar] [CrossRef]
- Sundaram, M.; Yao, S.Y.M.; Ng, A.M.L.; Griffiths, M.; Cass, C.E.; Baldwin, S.A.; Young, J.D. Chimeric Constructs between Human and Rat Equilibrative Nucleoside Transporters (hENT1 and rENT1) Reveal hENT1 Structural Domains Interacting with Coronary Vasoactive Drugs. J. Biol. Chem. 1998, 273, 21519–21525. [Google Scholar] [CrossRef] [Green Version]
- SenGupta, D.J.; Lum, P.Y.; Lai, Y.; Shubochkina, E.; Bakken, A.H.; Schneider, G.; Unadkat, J.D. A single glycine mutation in the equilibrative nucleoside transporter gene, hENT1, alters nucleoside transport activity and sensitivity to nitrobenzylthioinosine. Biochemistry 2002, 41, 1512–1519. [Google Scholar] [CrossRef]
- Leabman, M.K.; Huang, C.C.; DeYoung, J.; Carlson, E.J.; Taylor, T.R.; de la Cruz, M.; Johns, S.J.; Stryke, D.; Kawamoto, M.; Urban, T.J.; et al. Natural variation in human membrane transporter genes reveals evolutionary and functional constraints. Proc. Natl. Acad. Sci. USA 2003, 100, 5896–5901. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.N.; Goyal, R.K.; Roy, J.D.; Fairfull, L.D.; Wilson, J.W.; Ferrell, R.E. Functional single nucleotide polymorphism haplotypes in the human equilibrative nucleoside transporter 1. Pharmacogenet. Genom. 2006, 16, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.A.; Wang, L.Z.; Ng, S.S.; Chong, P.Y.; Yong, W.P.; Lee, S.C.; Liu, J.J.; Choo, T.B.; Tham, L.S.; Lee, H.S.; et al. Distribution of gemcitabine pathway genotypes in ethnic Asians and their association with outcome in non-small cell lung cancer patients. Lung Cancer 2009, 63, 121–127. [Google Scholar] [CrossRef]
- Gomez, A.; Ingelman-Sundberg, M. Pharmacoepigenetics: Its role in interindividual differences in drug response. Clin. Pharmacol. Ther. 2009, 85, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Manuyakorn, A.; Paulus, R.; Farrell, J.; Dawson, N.A.; Tze, S.; Cheung-Lau, G.; Hines, O.J.; Reber, H.; Seligson, D.B.; Horvath, S.; et al. Cellular histone modification patterns predict prognosis and treatment response in resectable pancreatic adenocarcinoma: Results from RTOG 9704. J. Clin. Oncol. 2010, 28, 1358–1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candelaria, M.; de la Cruz-Hernandez, E.; Taja-Chayeb, L.; Perez-Cardenas, E.; Trejo-Becerril, C.; Gonzalez-Fierro, A.; Chavez-Blanco, A.; Soto-Reyes, E.; Dominguez, G.; Trujillo, J.E.; et al. DNA Methylation-Independent Reversion of Gemcitabine Resistance by Hydralazine in Cervical Cancer Cells. PLoS ONE 2012, 7, e29181. [Google Scholar] [CrossRef]
- Calin, G.A.; Croce, C.M. MicroRNA signatures in human cancers. Nat. Rev. Cancer 2006, 6, 857–866. [Google Scholar] [CrossRef]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; DeSano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef]
- Lee, E.J.; Gusev, Y.; Jiang, J.; Nuovo, G.J.; Lerner, M.R.; Frankel, W.L.; Morgan, D.L.; Postier, R.G.; Brackett, D.J.; Schmittgen, T.D. Expression profiling identifies microRNA signature in pancreatic cancer. Int. J. Cancer 2006, 120, 1046–1054. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.-F.; Hannafon, B.N.; Zhao, Y.D.; Postier, R.G.; Ding, W.-Q. Plasma exosome miR-196a and miR-1246 are potential indicators of localized pancreatic cancer. Oncotarget 2017, 8, 77028–77040. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Xia, M.; Wu, F.; Ding, J.; Jiao, Y.; Zhan, Q.; An, F. Upregulated exosomic miR-23b-3p plays regulatory roles in the progression of pancreatic cancer. Oncol. Rep. 2017, 38, 2182–2188. [Google Scholar] [CrossRef] [Green Version]
- Mikamori, M.; Yamada, D.; Eguchi, H.; Hasegawa, S.; Kishimoto, T.; Tomimaru, Y.; Asaoka, T.; Noda, T.; Wada, H.; Kawamoto, K.; et al. MicroRNA-155 Controls Exosome Synthesis and Promotes Gemcitabine Resistance in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2017, 7, 42339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, L.; Ischenko, I.; Bao, Q.; Schwarz, B.; Nieß, H.; Wang, Y.; Renner, A.; Mysliwietz, J.; Jauch, K.-W.; et al. Antisense inhibition of microRNA-21 and microRNA-221 in tumor-initiating stem-like cells modulates tumorigenesis, metastasis, and chemotherapy resistance in pancreatic cancer. Targeted Oncol. 2015, 10, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.; Honselmann, K.; Liss, A. The Extracellular Matrix and Pancreatic Cancer: A Complex Relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.K. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science 2005, 307, 5758–5762. [Google Scholar] [CrossRef]
- Koong, A.C.; Mehta, V.K.; Le, Q.T.; Fisher, G.A.; Terris, D.J.; Brown, J.M.; Bastidas, A.J.; Vierra, M. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 919–922. [Google Scholar] [CrossRef]
- Büchler, P.; Reber, H.A.; Büchler, M.; Shrinkante, S.; Büchler, M.W.; Friess, H.; Semenza, G.L.; Hines, O.J. Hypoxia-inducible factor 1 regulates vascular endothelial growth factor expression in human pancreatic cancer. Pancreas 2003, 26, 56–64. [Google Scholar] [CrossRef]
- Shibaji, T.; Nagao, M.; Ikeda, N.; Kanehiro, H.; Hisanaga, M.; Ko, S.; Fukumoto, A.; Nakajima, Y. Prognostic significance of HIF-1 alpha overexpression in human pancreatic cancer. Anticancer Res. 2003, 23, 4721–4727. [Google Scholar]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltzschig, H.K.; Abdulla, P.; Hoffman, E.; Hamilton, K.E.; Daniels, D.; Schönfeld, C.; Löffler, M.; Reyes, G.; Duszenko, M.; Karhausen, J.; et al. HIF-1–dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J. Exp. Med. 2005, 202, 1493–1505. [Google Scholar] [CrossRef]
- Löffler, M.; Morote-Garcia, J.C.; Eltzschig, S.A.; Coe, I.R.; Eltzschig, H.K. Physiological roles of vascular nucleoside transporters. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1004–1013. [Google Scholar] [CrossRef] [Green Version]
- Dalin, S.; Sullivan, M.R.; Lau, A.N.; Grauman-Boss, B.; Mueller, H.S.; Kreidl, E.; Fenoglio, S.; Luengo, A.; Lees, J.A.; Vander Heiden, M.G.; et al. Deoxycytidine Release from Pancreatic Stellate Cells Promotes Gemcitabine Resistance. Cancer Res. 2019, 79, 5723–5733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Che, P.P.; Gregori, A.; Firuzi, O.; Dahele, M.; Sminia, P.; Peters, G.J.; Giovannetti, E. Pancreatic cancer resistance conferred by stellate cells: Looking for new preclinical models. Exp. Hematol. Oncol. 2020, 9, 18. [Google Scholar] [CrossRef]
- Mendez, M.G.; Restle, D.; Janmey, P.A. Vimentin enhances cell elastic behavior and protects against compressive stress. Biophys. J. 2014, 107, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Koay, E.J.; Zhang, W.; Qin, L.; Kirui, D.K.; Hussain, F.; Shen, H.; Ferrari, M. Human equilibrative nucleoside transporter-1 knockdown tunes cellular mechanics through epithelial-mesenchymal transition in pancreatic cancer cells. PLoS ONE 2014, 9, e107973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, T.; Li, H.-Y.; Wu, J.-S.; Niu, Q.; Duan, W.-H.; Han, Q.-Z.; Ji, W.-M.; Zhang, T.; Lv, W. Astaxanthin inhibits gemcitabine-resistant human pancreatic cancer progression through EMT inhibition and gemcitabine resensitization. Oncol. Lett. 2017, 14, 5400–5408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Wang, Y.; Zhang, W.-L.; Dong, M.-S.; Zhang, J.-H. Sclareolide enhances gemcitabine-induced cell death through mediating the NICD and Gli1 pathways in gemcitabine-resistant human pancreatic cancer. Mol. Med. Rep. 2017, 15, 1461–1470. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, F. MIR-26b regulates invasion and migration of lung cancer cells through targeting hENTI depending on RhoA/ROCK-I pathway. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017, 42, 755–761. [Google Scholar] [CrossRef]
- Hesler, R.A.; Huang, J.J.; Starr, M.D.; Treboschi, V.M.; Bernanke, A.G.; Nixon, A.B.; McCall, S.J.; White, R.R.; Blobe, G.C. TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in pancreatic ductal adenocarcinoma through downregulation of the nucleoside transporters hENT1 and hCNT3. Carcinogenesis 2016, 37, 1041–1051. [Google Scholar] [CrossRef] [Green Version]
- Le Large, T.Y.S.; El Hassouni, B.; Funel, N.; Kok, B.; Piersma, S.R.; Pham, T.V.; Olive, K.P.; Kazemier, G.; van Laarhoven, H.W.M.; Jimenez, C.R.; et al. Proteomic analysis of gemcitabine-resistant pancreatic cancer cells reveals that microtubule-associated protein 2 upregulation associates with taxane treatment. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Nivillac, N.M.I.; Bacani, J.; Coe, I.R. The life cycle of human equilibrative nucleoside transporter 1: From ER export to degradation. Exp. Cell Res. 2011, 317, 1567–1579. [Google Scholar] [CrossRef]
- Patel, G.K.; Khan, M.A.; Bhardwaj, A.; Srivastava, S.K.; Zubair, H.; Patton, M.C.; Singh, S.; Khushman, M.; Singh, A.P. Exosomes confer chemoresistance to pancreatic cancer cells by promoting ROS detoxification and miR-155-mediated suppression of key gemcitabine-metabolising enzyme, DCK. Br. J. Cancer 2017, 116, 609–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergman, A.M.; Adema, A.D.; Balzarini, J.; Bruheim, S.; Fichtner, I.; Noordhuis, P.; Fodstad, Ø.; Myhren, F.; Sandvold, M.L.; Hendriks, H.R.; et al. Antiproliferative activity, mechanism of action and oral antitumor activity of CP-4126, a fatty acid derivative of gemcitabine, in in vitro and in vivo tumor models. Invest. New Drugs 2011, 29, 456–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adema, A.D.; Smid, K.; Losekoot, N.; Honeywell, R.J.; Verheul, H.M.; Myhren, F.; Sandvold, M.L.; Peters, G.J. Metabolism and accumulation of the lipophilic deoxynucleoside analogs elacytarabine and CP-4126. Investig. New Drugs 2012, 30, 1908–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzuto, I.; Ghazaly, E.; Peters, G.J. Pharmacological factors affecting accumulation of gemcitabine’s active metabolite, gemcitabine triphosphate. Pharmacogenomics 2017, 18, 911–925. [Google Scholar] [CrossRef]
- Matsumura, N.; Nakamura, Y.; Kohjimoto, Y.; Inagaki, T.; Nanpo, Y.; Yasuoka, H.; Ohashi, Y.; Hara, I. The prognostic significance of human equilibrative nucleoside transporter 1 expression in patients with metastatic bladder cancer treated with gemcitabine-cisplatin-based combination chemotherapy. BJU Int. 2011, 108, E110–E116. [Google Scholar] [CrossRef]
- Santini, D.; Schiavon, G.; Vincenzi, B.E.; Cass, C.; Vasile, E.D.; Manazza, A.; Catalano, V.G.; Baldi, G.; Lai, R.; Rizzo, S.; et al. Human Equilibrative Nucleoside Transporter 1 (hENT1) Levels Predict Response to Gemcitabine in Patients With Biliary Tract Cancer (BTC). Curr. Cancer Drug Targets 2011, 11, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Oguri, T.; Achiwa, H.; Muramatsu, H.; Ozasa, H.; Sato, S.; Shimizu, S.; Yamazaki, H.; Eimoto, T.; Ueda, R. The absence of human equilibrative nucleoside transporter 1 expression predicts nonresponse to gemcitabine-containing chemotherapy in non-small cell lung cancer. Cancer Lett. 2007, 256, 112–119. [Google Scholar] [CrossRef]
- Oba, A.; Ho, F.; Bao, Q.R.; Al-Musawi, M.H.; Schulick, R.D.; Del Chiaro, M. Neoadjuvant Treatment in Pancreatic Cancer. Front. Oncol. 2020, 10, 245. [Google Scholar] [CrossRef]




| microRNA | microRNA acc | Experiment Type | Database Source | Comments | Reference |
|---|---|---|---|---|---|
| hsa-miR-196a-3p | MIMAT0004562 | PAR-CLIP | mirtarbase | Up-regulated in exosomes of PDAC’s serum | [67] |
| hsa-miR-221-5p | MIMAT0004568 | Degradome sequencing | tarbase | Up-regulated in PDAC cancer stem cells | [70] |
| hsa-miR-23b-3p | MIMAT0000418 | Degradome sequencing | tarbase | Up-regulated in exosomes of PDAC’s serum and correlated to CA19–9 | [68] |
| hsa-miR-155-5p | MIMAT0000646 | Degradome sequencing | tarbase | Up-regulated in GEM resistant PDAC cells | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Randazzo, O.; Papini, F.; Mantini, G.; Gregori, A.; Parrino, B.; Liu, D.S.K.; Cascioferro, S.; Carbone, D.; Peters, G.J.; Frampton, A.E.; et al. “Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer. Cancers 2020, 12, 3206. https://doi.org/10.3390/cancers12113206
Randazzo O, Papini F, Mantini G, Gregori A, Parrino B, Liu DSK, Cascioferro S, Carbone D, Peters GJ, Frampton AE, et al. “Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer. Cancers. 2020; 12(11):3206. https://doi.org/10.3390/cancers12113206
Chicago/Turabian StyleRandazzo, Ornella, Filippo Papini, Giulia Mantini, Alessandro Gregori, Barbara Parrino, Daniel S. K. Liu, Stella Cascioferro, Daniela Carbone, Godefridus J. Peters, Adam E. Frampton, and et al. 2020. "“Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer" Cancers 12, no. 11: 3206. https://doi.org/10.3390/cancers12113206
APA StyleRandazzo, O., Papini, F., Mantini, G., Gregori, A., Parrino, B., Liu, D. S. K., Cascioferro, S., Carbone, D., Peters, G. J., Frampton, A. E., Garajova, I., & Giovannetti, E. (2020). “Open Sesame?”: Biomarker Status of the Human Equilibrative Nucleoside Transporter-1 and Molecular Mechanisms Influencing its Expression and Activity in the Uptake and Cytotoxicity of Gemcitabine in Pancreatic Cancer. Cancers, 12(11), 3206. https://doi.org/10.3390/cancers12113206