Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Manufacturing Process
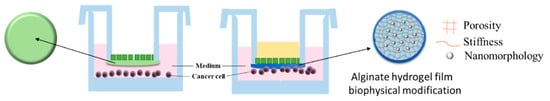
2.1.1. Manufacturing of Transwell Alginate Hydrogel-TAH
2.1.2. Developing a Biophysical Cell Homing Model with Alginate Hydrogel
2.2. Biophysical Cues and Cell Behaviors
2.2.1. TAH as a Molecular Sieving Substrate to Attract Cancer Cells via Growth Factors
2.2.2. Controlling TAH Stiffness by Ethanol for Promoting Physicotactic Cell Migration
2.2.3. The Modification of Hydrogel Topography to Alter the Homing Behavior of Cells
2.3. Gene Analysis
Physical Cues of TAH Model Regulate Stemness-Dependent Homing of Breast Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Materials for Hydrogel Curing
4.2. Morphological Characteristics of Alginate Hydrogel Films
4.2.1. Scanning Electron Microscope (SEM)
4.2.2. In-Situ SEM Scanning of Liquid Samples
4.3. Mechanical Compression Test
4.4. Cell Culture
4.5. Nucleus Localization of Cells Attached on TAH Using Fluorescence Dye
4.6. Determination of The Number of Viable Cells
4.7. Attenuated Total Reflection Fourier Transform Infrared (FTIR) Spectroscopy
4.8. Laser-Scanning Confocal Microscopy Analysis
4.9. RNA Isolation and Real-Time PCR (qRT-PCR)
4.10. Immunofluorescence Staining for Stem Cell Markers
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogenrieder, T.; Herlyn, M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene 2003, 22, 6524–6536. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.P.; Massagué, J. Cancer metastasis: Building a framework. Cell 2006, 127, 679–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Trier, S.M.; Keely, P.J. Contact guidance mediated three-dimensional cell migration is regulated by Rho/ROCK-dependent matrix reorganization. Biophys. J. 2008, 95, 5374–5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilkes, D.M.; Semenza, G.L.; Wirtz, D. Hypoxia and the extracellular matrix: Drivers of tumour metastasis. Nat. Rev. Cancer 2014, 14, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedl, P.; Alexander, S. Cancer invasion and the microenvironment: Plasticity and reciprocity. Cell 2011, 147, 992–1009. [Google Scholar] [CrossRef] [Green Version]
- Malik, R.; Lelkes, P.I.; Cukierman, E. Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends Biotechnol. 2015, 33, 230–236. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, H.; Sahai, E. Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 2018, 20, 766. [Google Scholar] [CrossRef]
- Suresh, S. Biomechanics and biophysics of cancer cells. Acta Mater. 2007, 55, 3989–4014. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Mouw, J.K.; Weaver, V.M. Forcing form and function: Biomechanical regulation of tumor evolution. Trends Cell Biol. 2011, 21, 47–56. [Google Scholar] [CrossRef] [Green Version]
- Tracqui, P. Biophysical models of tumour growth. Rep. Prog. Phys. 2009, 72, 056701. [Google Scholar] [CrossRef] [Green Version]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kariri, Y.A.; Aleskandarany, M.A.; Joseph, C.; Kurozumi, S.; Mohammed, O.J.; Toss, M.S.; Green, A.R.; Rakha, E.A. Molecular Complexity of Lymphovascular Invasion: The Role of Cell Migration in Breast Cancer as a Prototype. Pathobiology 2020, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Senthebane, D.A.; Jonker, T.; Rowe, A.; Thomford, N.E.; Munro, D.; Dandara, C.; Wonkam, A.; Govender, D.; Calder, B.; Soares, N.C. The role of tumor microenvironment in chemoresistance: 3D extracellular matrices as accomplices. Int. J. Mol. Sci. 2018, 19, 2861. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Weaver, V.M. Mechanics, malignancy, and metastasis: The force journey of a tumor cell. Cancer Metastasis Rev. 2009, 28, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Pathak, A.; Kumar, S. Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc. Natl. Acad. Sci. USA 2012, 109, 10334–10339. [Google Scholar] [CrossRef] [Green Version]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Nelson, C.M.; Bissell, M.J. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 2006, 22, 287–309. [Google Scholar] [CrossRef] [Green Version]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [Green Version]
- Balzer, E.M.; Tong, Z.; Paul, C.D.; Hung, W.C.; Stroka, K.M.; Boggs, A.E.; Martin, S.S.; Konstantopoulos, K. Physical confinement alters tumor cell adhesion and migration phenotypes. FASEB J. 2012, 26, 4045–4056. [Google Scholar] [CrossRef] [Green Version]
- Carey, S.P.; Goldblatt, Z.E.; Martin, K.E.; Romero, B.; Williams, R.M.; Reinhart-King, C.A. Local extracellular matrix alignment directs cellular protrusion dynamics and migration through Rac1 and FAK. Integr. Biol. 2016, 8, 821–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Rev. Cancer 2017, 17, 659. [Google Scholar] [CrossRef] [PubMed]
- Vargas, D.A.; Gonçalves, I.G.; Heck, T.; Smeets, B.; Lafuente-Gracia, L.; Ramon, H.; Van Oosterwyck, H. Modeling of Mechanosensing Mechanisms Reveals Distinct Cell Migration Modes to Emerge From Combinations of Substrate Stiffness and Adhesion Receptor–Ligand Affinity. Front. Bioeng. Biotechnol. 2020, 8, 459. [Google Scholar] [CrossRef]
- Loebel, C.; Mauck, R.L.; Burdick, J.A. Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nat. Mater. 2019, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.D.; Yamada, K.M. Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp. Cell Res. 2016, 343, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, V.; Mythreye, K.; O’Brien, E.T.; Berchuck, A.; Blobe, G.C.; Superfine, R. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res. 2011, 71, 5075–5080. [Google Scholar] [CrossRef] [Green Version]
- Cavo, M.; Fato, M.; Peñuela, L.; Beltrame, F.; Raiteri, R.; Scaglione, S. Microenvironment complexity and matrix stiffness regulate breast cancer cell activity in a 3D in vitro model. Sci. Rep. 2016, 6, 35367. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, O.; Koshy, S.T.; Da Cunha, C.B.; Shin, J.-W.; Verbeke, C.S.; Allison, K.H.; Mooney, D.J. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nat. Mater. 2014, 13, 970–978. [Google Scholar] [CrossRef]
- Kuo, C.H.R.; Xian, J.; Brenton, J.D.; Franze, K.; Sivaniah, E. Complex stiffness gradient substrates for studying mechanotactic cell migration. Adv. Mater. 2012, 24, 6059–6064. [Google Scholar] [CrossRef]
- Reinhard, J.; Brösicke, N.; Theocharidis, U.; Faissner, A. The extracellular matrix niche microenvironment of neural and cancer stem cells in the brain. Int. J. Biochem. Cell Biol. 2016, 81, 174–183. [Google Scholar] [CrossRef]
- Vlashi, E.; Pajonk, F. Cancer stem cells, cancer cell plasticity and radiation therapy. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2015; pp. 28–35. [Google Scholar]
- Senthebane, D.A.; Rowe, A.; Thomford, N.E.; Shipanga, H.; Munro, D.; Al Mazeedi, M.A.; Almazyadi, H.A.; Kallmeyer, K.; Dandara, C.; Pepper, M.S. The role of tumor microenvironment in chemoresistance: To survive, keep your enemies closer. Int. J. Mol. Sci. 2017, 18, 1586. [Google Scholar] [CrossRef] [PubMed]
- Shamsian, A.; Sepand, M.R.; Kachousangi, M.J.; Dara, T.; Ostad, S.N.; Atyabi, F.; Ghahremani, M.H. Targeting Tumorigenicity of Breast Cancer Stem Cells Using SAHA/Wnt-b Catenin Antagonist Loaded Onto Protein Corona of Gold Nanoparticles. Int. J. Nanomed. 2020, 15, 4063–4078. [Google Scholar] [CrossRef]
- Brown, Y.; Hua, S.; Tanwar, P.S. Extracellular matrix-mediated regulation of cancer stem cells and chemoresistance. Int. J. Biochem. Cell Biol. 2019, 109, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Boman, B.M.; Wicha, M.S.; PETRELLI, N.J. Cancer Stem Cells. J. Clin. Oncol. 2008, 26, 135–140. [Google Scholar]
- Kelly, S.E.; Di Benedetto, A.; Greco, A.; Howard, C.M.; Sollars, V.E.; Primerano, D.A.; Valluri, J.V.; Claudio, P.P. Rapid selection and proliferation of CD133 (+) cells from cancer cell lines: Chemotherapeutic implications. PLoS ONE 2010, 5, e10035. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kotliarova, S.; Kotliarov, Y.; Li, A.; Su, Q.; Donin, N.M.; Pastorino, S.; Purow, B.W.; Christopher, N.; Zhang, W. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell 2006, 9, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. AMS 2018, 14, 910. [Google Scholar] [CrossRef]
- Zhong, J.; Baquiran, J.B.; Bonakdar, N.; Lees, J.; Ching, Y.W.; Pugacheva, E.; Fabry, B.; O’Neill, G.M. NEDD9 stabilizes focal adhesions, increases binding to the extra-cellular matrix and differentially effects 2D versus 3D cell migration. PLoS ONE 2012, 7, e35058. [Google Scholar] [CrossRef] [Green Version]
- Pickl, M.; Ries, C. Comparison of 3D and 2D tumor models reveals enhanced HER2 activation in 3D associated with an increased response to trastuzumab. Oncogene 2009, 28, 461. [Google Scholar] [CrossRef] [Green Version]
- David, L.; Dulong, V.; Le Cerf, D.; Chauzy, C.; Norris, V.; Delpech, B.; Lamacz, M.; Vannier, J.-P. Reticulated hyaluronan hydrogels: A model for examining cancer cell invasion in 3D. Matrix Biol. 2004, 23, 183–193. [Google Scholar] [CrossRef]
- Fischbach, C.; Chen, R.; Matsumoto, T.; Schmelzle, T.; Brugge, J.S.; Polverini, P.J.; Mooney, D.J. Engineering tumors with 3D scaffolds. Nat. Methods 2007, 4, 855. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loessner, D.; Stok, K.S.; Lutolf, M.P.; Hutmacher, D.W.; Clements, J.A.; Rizzi, S.C. Bioengineered 3D platform to explore cell–ECM interactions and drug resistance of epithelial ovarian cancer cells. Biomaterials 2010, 31, 8494–8506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Xiao, Z.; Meng, Y.; Zhao, Y.; Han, J.; Su, G.; Chen, B.; Dai, J. The enhancement of cancer stem cell properties of MCF-7 cells in 3D collagen scaffolds for modeling of cancer and anti-cancer drugs. Biomaterials 2012, 33, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.; Anuradha, E.; Solomon, F.P. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Pang, X.; O’Malley, C.; Borges, J.; Rahman, M.M.; Collis, D.W.; Mano, J.F.; Mackenzie, I.C.; Azevedo, H.S. Supramolecular Presentation of Hyaluronan onto Model Surfaces for Studying the Behavior of Cancer Stem Cells. Adv. Biosyst. 2019, 3, 1900017. [Google Scholar] [CrossRef]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Lynch, M.E.; Brooks, D.; Mohanan, S.; Lee, M.J.; Polamraju, P.; Dent, K.; Bonassar, L.J.; van der Meulen, M.C.; Fischbach, C. In Vivo tibial compression decreases osteolysis and tumor formation in a human metastatic breast cancer model. J. Bone Miner. Res. 2013, 28, 2357–2367. [Google Scholar] [CrossRef]
- Lombardo, Y.; Filipović, A.; Molyneux, G.; Periyasamy, M.; Giamas, G.; Hu, Y.; Trivedi, P.S.; Wang, J.; Yagüe, E.; Michel, L. Nicastrin regulates breast cancer stem cell properties and tumor growth in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 16558–16563. [Google Scholar] [CrossRef] [Green Version]
- Maliszewska-Olejniczak, K.; Brodaczewska, K.K.; Bielecka, Z.F.; Solarek, W.; Kornakiewicz, A.; Szczylik, C.; Porta, C.; Czarnecka, A.M. Development of extracellular matrix supported 3D culture of renal cancer cells and renal cancer stem cells. Cytotechnology 2019, 71, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, M.C.; Foley, M.A.; Cardinal, K.O.H. Thinking inside the box: Keeping tissue-engineered constructs in vitro for use as preclinical models. Tissue Eng. Part B Rev. 2013, 19, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Horch, R.E.; Boos, A.M.; Quan, Y.; Bleiziffer, O.; Detsch, R.; Boccaccini, A.R.; Alexiou, C.; Sun, J.; Beier, J.P.; Arkudas, A. Cancer research by means of tissue engineering—Is there a rationale? J. Cell. Mol. Med. 2013, 17, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Burdett, E.; Kasper, F.K.; Mikos, A.G.; Ludwig, J.A. Engineering tumors: A tissue engineering perspective in cancer biology. Tissue Eng. Part B Rev. 2010, 16, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.-J.; Lee, K.Y.; Mooney, D.J. Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration. Polymer 2002, 43, 6239–6246. [Google Scholar] [CrossRef]
- Mano, J.; Silva, G.; Azevedo, H.S.; Malafaya, P.; Sousa, R.; Silva, S.S.; Boesel, L.; Oliveira, J.M.; Santos, T.; Marques, A. Natural origin biodegradable systems in tissue engineering and regenerative medicine: Present status and some moving trends. J. R. Soc. Interface 2007, 4, 999–1030. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A. Cell Culture: Biology’s New Dimension. Nature 2003, 424, 870–872. [Google Scholar] [CrossRef]
- Sunyer, R.; Jin, A.J.; Nossal, R.; Sackett, D.L. Fabrication of hydrogels with steep stiffness gradients for studying cell mechanical response. PLoS ONE 2012, 7, e46107. [Google Scholar] [CrossRef] [Green Version]
- Hadjipanayi, E.; Mudera, V.; Brown, R. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009, 3, 77–84. [Google Scholar] [CrossRef]
- Maia, F.R.; Fonseca, K.B.; Rodrigues, G.; Granja, P.L.; Barrias, C.C. Matrix-driven formation of mesenchymal stem cell–extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 2014, 10, 3197–3208. [Google Scholar] [CrossRef]
- Peerani, R.; Zandstra, P.W. Enabling stem cell therapies through synthetic stem cell–niche engineering. J. Clin. Investig. 2010, 120, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguado, B.A.; Bushnell, G.G.; Rao, S.S.; Jeruss, J.S.; Shea, L.D. Engineering the pre-metastatic niche. Nat. Biomed. Eng. 2017, 1, 0077. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.N.; Singh, A.; Rothenberg, A.R.; Elisseeff, J.H.; Ewald, A.J. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials 2013, 34, 9486–9495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J. Transwell® invasion assays. In Cell Migration; Springer: Berlin/Heidelberg, Germany, 2011; pp. 97–110. [Google Scholar]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In Vitro cell migration and invasion assays. J. Vis. Exp. 2014, 88, e51046. [Google Scholar]
- Venkatesan, J.; Bhatnagar, I.; Manivasagan, P.; Kang, K.-H.; Kim, S.-K. Alginate composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2015, 72, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Bidarra, S.J.; Barrias, C.C.; Granja, P.L. Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater. 2014, 10, 1646–1662. [Google Scholar] [CrossRef]
- Lieleg, O.; Ribbeck, K. Biological hydrogels as selective diffusion barriers. Trends Cell Biol. 2011, 21, 543–551. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.S.; Adekoya, D.; Enenmoh, I.; Clarke, O.; Wang, P.; Sarkyssian, M.; Wu, Y.; Vadgama, J.V. Salinomycin abolished STAT3 and STAT1 interactions and reduced telomerase activity in colorectal cancer cells. Anticancer Res. 2017, 37, 445–453. [Google Scholar] [CrossRef]
- Zaman, M.H.; Trapani, L.M.; Sieminski, A.L.; MacKellar, D.; Gong, H.; Kamm, R.D.; Wells, A.; Lauffenburger, D.A.; Matsudaira, P. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc. Natl. Acad. Sci. USA 2006, 103, 10889–10894. [Google Scholar] [CrossRef] [Green Version]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Cukierman, E.; Bassi, D.E. Physico-mechanical aspects of extracellular matrix influences on tumorigenic behaviors. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2010; Volume 20, pp. 139–145. [Google Scholar]
- Sheen-Chen, S.-M.; Chen, W.-J.; Eng, H.-L.; Chou, F.-F. Serum concentration of tumor necrosis factor in patients with breast cancer. Breast Cancer Res. Treat. 1997, 43, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; McDowell, S.A. Immunological regulation of vascular inflammation during cancer metastasis. Front. Immunol. 2019, 10, 1984. [Google Scholar]
- Riching, K.M.; Cox, B.L.; Salick, M.R.; Pehlke, C.; Riching, A.S.; Ponik, S.M.; Bass, B.R.; Crone, W.C.; Jiang, Y.; Weaver, A.M. 3D collagen alignment limits protrusions to enhance breast cancer cell persistence. Biophys. J. 2014, 107, 2546–2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.; Liphardt, J.; Hwang, E.; Weaver, V. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraning-Rush, C.M.; Califano, J.P.; Reinhart-King, C.A. Cellular traction stresses increase with increasing metastatic potential. PLoS ONE 2012, 7, e32572. [Google Scholar] [CrossRef] [Green Version]
- Yim, E.K.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef]
- Groth, T.; Altankov, G. Fibroblast spreading and proliferation on hydrophilic and hydrophobic surfaces is related to tyrosine phosphorylation in focal contacts. J. Biomater. Sci. Polym. Ed. 1996, 7, 297–305. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139. [Google Scholar] [CrossRef] [Green Version]
- Kechagia, J.Z.; Ivaska, J.; Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019, 20, 457–473. [Google Scholar] [CrossRef]
- Wu, J.; Mao, Z.; Tan, H.; Han, L.; Ren, T.; Gao, C. Gradient biomaterials and their influences on cell migration. Interface Focus 2012, 2, 337–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, A.; Hall, C.; Barney, L.; Babbitt, C.; Peyton, S. Mechanosensing of Integrin α6 and EGFR Converges at Calpain 2. bioRxiv 2017, 164525. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Qu, J.; Huang, X.; Kurundkar, A.; Zhu, L.; Yang, N.; Venado, A.; Ding, Q.; Liu, G.; Antony, V.B. Mechanosensing by the α 6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis. Nat. Commun. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, C.M.; Van De Water, L. Integrin Regulation of CAF Differentiation and Function. Cancer 2019, 11, 715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Lin, M.; Huang, G.; Li, Y.; Wang, S.; Bai, G.; Lu, T.J.; Xu, F. 3D Spatiotemporal Mechanical Microenvironment: A Hydrogel-Based Platform for Guiding Stem Cell Fate. Adv. Mater. 2018, 30, 1705911. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Cancer metastases: Challenges and opportunities. Acta Pharm. Sin. B 2015, 5, 402–418. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Borgström, B.; Kempengren, S.; Persson, L.; Hegardt, C.; Strand, D.; Oredsson, S. Breast cancer stem cell selectivity of synthetic nanomolar-active salinomycin analogs. BMC Cancer 2016, 16, 145. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Wu, D.; Wang, Y.; Wang, Z.; Zou, C.; Dai, Y.; Ng, C.-F.; Teoh, J.Y.-C.; Chan, F.L. Development of a novel and economical agar-based non-adherent three-dimensional culture method for enrichment of cancer stem-like cells. Stem Cell Res. Ther. 2018, 9, 243. [Google Scholar] [CrossRef]
- Pisanu, M.E.; Noto, A.; De Vitis, C.; Masiello, M.G.; Coluccia, P.; Proietti, S.; Giovagnoli, M.R.; Ricci, A.; Giarnieri, E.; Cucina, A. Lung cancer stem cell lose their stemness default state after exposure to microgravity. BioMed Res. Int. 2014, 2014, 18–20. [Google Scholar] [CrossRef]
- Strnadel, J.; Woo, S.M.; Choi, S.; Wang, H.; Grendar, M.; Fujimura, K. 3D Culture Protocol for Testing Gene Knockdown Efficiency and Cell Line Derivation. BIO-PROTOCOL 2018, 8, 78. [Google Scholar] [CrossRef]
- Bielecka, Z.F.; Maliszewska-Olejniczak, K.; Safir, I.J.; Szczylik, C.; Czarnecka, A.M. Three-dimensional cell culture model utilization in cancer stem cell research. Biol. Rev. 2017, 92, 1505–1520. [Google Scholar] [CrossRef] [PubMed]
- Tirino, V.; Desiderio, V.; Paino, F.; Papaccio, G.; De Rosa, M. Methods for cancer stem cell detection and isolation. In Somatic Stem Cells; Springer: Berlin/Heidelberg, Germany, 2012; pp. 513–529. [Google Scholar]
- Oshima, N.; Yamada, Y.; Nagayama, S.; Kawada, K.; Hasegawa, S.; Okabe, H.; Sakai, Y.; Aoi, T. Induction of cancer stem cell properties in colon cancer cells by defined factors. PLoS ONE 2014, 9, e101735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, Y.-C.; Hsiao, A.Y.; Allen, S.G.; Torisawa, Y.-S.; Ho, M.; Takayama, S. High-throughput 3D spheroid culture and drug testing using a 384 hanging drop array. Analyst 2011, 136, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, S.; Mehta, P.; Xie, Y.; Lei, Y.L.; Mehta, G. Ovarian cancer stem cells and macrophages reciprocally interact through the WNT pathway to promote pro-tumoral and malignant phenotypes in 3D engineered microenvironments. J. Immunother. Cancer 2019, 7, 190. [Google Scholar] [CrossRef] [Green Version]
- Bregenzer, M.E.; Davis, C.; Horst, E.N.; Mehta, P.; Novak, C.M.; Raghavan, S.; Snyder, C.S.; Mehta, G. Physiologic Patient Derived 3D Spheroids for Anti-neoplastic Drug Screening to Target Cancer Stem Cells. J. Vis. Exp. 2019, e59696. [Google Scholar] [CrossRef]
- Shigeto, K.; Masaki, Y.; Keita, S.; Masaya, Y.; Etsuko, F.; Kiyotaka, N.; Suzuki, M. Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Sci. Rep. Nat. Publ. Group 2020, 10, 3156. [Google Scholar]
- Izawa, Y.; Kashii-Magaribuchi, K.; Yoshida, K.; Nosaka, M.; Tsuji, N.; Yamamoto, A.; Kuroyanagi, K.; Tono, K.; Tanihata, M.; Imanishi, M. Stem-like human breast cancer cells initiate vasculogenic mimicry on matrigel. Acta Histochem. Cytochem. 2018, 51, 18041. [Google Scholar] [CrossRef] [Green Version]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.W.; Kelly, D.J. Cytochrome c biogenesis in C ampylobacter jejuni requires cytochrome c6 (CccA; C j1153) to maintain apocytochrome cysteine thiols in a reduced state for haem attachment. Mol. Microbiol. 2015, 96, 1298–1317. [Google Scholar]
- Li, Y.; Rogoff, H.A.; Keates, S.; Gao, Y.; Murikipudi, S.; Mikule, K.; Leggett, D.; Li, W.; Pardee, A.B.; Li, C.J. Suppression of cancer relapse and metastasis by inhibiting cancer stemness. Proc. Natl. Acad. Sci. USA 2015, 112, 1839–1844. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415. [Google Scholar] [CrossRef] [Green Version]







| Dyes | Diffusion Coefficient (mm2/s) ± SEM | |||
|---|---|---|---|---|
| kD | 2% TAH | 4% TAH | 6% TAH | |
| R6G | 479 | 6.20 × 10−5 ± 3.48 × 10−6 | 2.58 × 10−5 ± 1.43 × 10−6 | 2.40 × 10−5 ± 1.34 × 10−6 |
| FITC–Dextran | 20,000 | 1.96 × 10−5 ± 1.26 × 10−6 | 1.72 × 10−5 ± 7.79 × 10−7 | 1.19 × 10−5 ± 7.76 × 10−7 |
| Rhodamine B | 70,000 | 6.28 × 10−7 ± 2.35 × 10−7 | 3.90 × 10−7 ± 3.06 × 10−7 | 8.48 × 10−7 ± 2.33 × 10−7 |
| Technology | Cost | Time Required for a Culture | Operational Complexity | Strengths | Weaknesses |
|---|---|---|---|---|---|
| TAH | Very low | 1 day | simple | Simple procedure; Microenvironment mimicking | Not reusable |
| 3D culture in ultra-low attachment (ULA) plates | High | 10–14 days | simple | Ready to use; long-term storage | Not reusable, costly, heterogeneous size [91,92,93] |
| Hanging drop | Low | 5–7 days | Labor-intensive | Controllable sphere size, High-throughput production | specific cultureware, short-term culturing, difficult to replace medium [97,98,99] |
| Gels for 3D cultures—e.g., Matrigel | High | 2–3 days | Labor-intensive | Microenvironment mimicking | Costly, temperature sensitive, complicated operation, difficulty in cell isolation [50,100,101] |
| Gene | Primer Sequences | |
|---|---|---|
| Target | Forward Primer | Reverse Primer |
| CD133 | 5′-TCCACAGAAATTTACCTACATTGG-3′ | 5′-CAGCAGAGAGCAGATGACCA-3′ |
| OCT4 | 5′-CTTGCTGCAGAAGTGGGTGGAGGAA-3 | 5′-CTGCAGTGTGGGTTTCGGGCA-3′ |
| SOX2 | 5′-AAATGGGAGGGGTGCAAAAGAGGAG-3′ | 5′-CAGCTGTCATTTGCTGTGGGTGATG-3′ |
| GAPDH | 5′-TGAAGGTCGGAGTCAACGGATT-3′ | 5′-CCTGGAAGATGGTGATGGGATT-3′ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, H.-Y.; Chen, Y.-J.; Hsu, C.-J.; Liu, Y.-W.; Chiou, J.-F.; Lu, L.-S.; Tseng, F.-G. Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells. Cancers 2020, 12, 2176. https://doi.org/10.3390/cancers12082176
Chu H-Y, Chen Y-J, Hsu C-J, Liu Y-W, Chiou J-F, Lu L-S, Tseng F-G. Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells. Cancers. 2020; 12(8):2176. https://doi.org/10.3390/cancers12082176
Chicago/Turabian StyleChu, Hsueh-Yao, Yin-Ju Chen, Chun-Jieh Hsu, Yang-Wei Liu, Jeng-Fong Chiou, Long-Sheng Lu, and Fan-Gang Tseng. 2020. "Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells" Cancers 12, no. 8: 2176. https://doi.org/10.3390/cancers12082176
APA StyleChu, H. -Y., Chen, Y. -J., Hsu, C. -J., Liu, Y. -W., Chiou, J. -F., Lu, L. -S., & Tseng, F. -G. (2020). Physical Cues in the Microenvironment Regulate Stemness-Dependent Homing of Breast Cancer Cells. Cancers, 12(8), 2176. https://doi.org/10.3390/cancers12082176