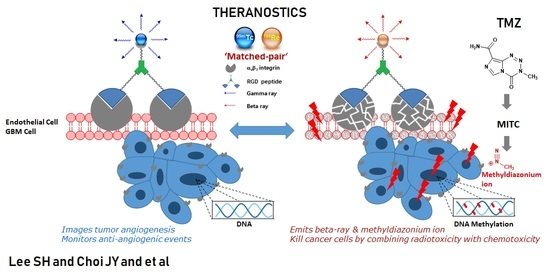
Effect of Peptide Receptor Radionuclide Therapy in Combination with Temozolomide against Tumor Angiogenesis in a Glioblastoma Model
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Treatments
2.3. Preparation of Tumor-Bearing Mice
2.4. Radiotherapy for the U87-MG Xenografts
2.5. Combination Therapy of TMZ with 188Re-IDA-D-[c(RGDfK)]2 in the U87-MG Xenografts
2.6. Immunohistochemistry
2.7. Confocal Microscopy
2.8. Tumor Growth Measurement
2.9. SPECT Image Analysis
2.10. Statistical Analysis
3. Results
3.1. Pharmacokinetic Studies of 99mTc-IDA-D-[c(RGDfK)]2 in U87-MG Xenografts
3.2. Anti-Angiogenic Effect of 188Re-IDA-D-[c(RGDfK)]2
3.3. Selectivity of Radiotherapy
3.4. Theranostics for Tumor Angiogenesis
3.5. PRRT Combined with TMZ
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Backer, M.V.; Backer, J.M. Imaging Key Biomarkers of Tumor Angiogenesis. Theranostics 2012, 2, 502–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Brooks, P.C.; Montgomery, A.M.P.; Rosenfeld, M.; Reisfeld, R.A.; Hu, T.; Klier, G.; Cheresh, D.A. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell 1994, 79, 1157–1164. [Google Scholar] [CrossRef]
- Kerbel, R.; Folkman, J. Clinical translation of angiogenesis inhibitors. Nat. Rev. Cancer 2002, 2, 727–739. [Google Scholar] [CrossRef]
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef]
- Haubner, R.; Wester, H.J.; Weber, W.A.; Mang, C.; Ziegler, S.I.; Goodman, S.L.; Senekowitsch-Schmidtke, R.; Kessler, H.; Schwaiger, M. Noninvasive Imaging of αvβ3 Integrin Expression Using 18F-labeled RGD-containing Glycopeptide and Positron Emission Tomography. Cancer Res. 2001, 61, 1781–1785. [Google Scholar]
- Zhang, X.; Xiong, Z.; Wu, Y.; Cai, W.; Tseng, J.R.; Gambhir, S.S.; Chen, X. Quantitative PET Imaging of Tumor Integrin αvβ3 Expression with 18F-FRGD2. J. Nucl. Med. 2006, 47, 113–121. [Google Scholar]
- McParland, B.J.; Miller, M.P.; Spinks, T.J.; Kenny, L.M.; Osman, S.; Khela, M.K.; Aboagye, E.; Coombes, R.C.; Hui, A.M.; Cohen, P.S. The Biodistribution and Radiation Dosimetry of the Arg-Gly-Asp Peptide 18F-AH111585 in Healthy Volunteers. J. Nucl. Med. 2008, 49, 1664–1667. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Kim, Y.S.; Zhai, S.; Liu, Z.; Chen, X.; Liu, S. Improving Tumor Uptake and Pharmacokinetics of 64Cu-Labeled Cyclic RGD Peptide Dimers with Gly3 and PEG4 Linkers. Bioconjugate Chem. 2009, 20, 750–759. [Google Scholar] [CrossRef] [Green Version]
- Janssen, M.L.; Oyen, W.J.; Dijkgraaf, I.; Massuger, L.F.; Frielink, C.; Edwards, D.S.; Rajopadhye, M.; Boonstra, H.; Corstens, F.H.; Boerman, O.C. Tumor Targeting with Radiolabeled αvβ3 Integrin Binding Peptides in a Nude Mouse Model. Cancer Res. 2002, 62, 6146–6151. [Google Scholar]
- Lee, B.C.; Sung, H.J.; Kim, J.S.; Jung, K.H.; Choe, Y.S.; Lee, K.H.; Chi, D.Y. Synthesis of Tc-99m labeled glucosamino-Asp-cyclic(Arg-Gly-Asp-D-Phe-Lys) as a potential angiogenesis imaging agent. Bioorg. Med. Chem. 2007, 15, 7755–7764. [Google Scholar] [CrossRef]
- Indrevoll, B.; Kindberg, G.M.; Solbakken, M.; Bjurgert, E.; Johansen, J.H.; Karlsen, H.; Mendizabal, M.; Cuthbertson, A. NC-100717: A versatile RGD peptide scaffold for angiogenesis imaging. Bioorg. Med. Chem. Lett. 2006, 16, 6190–6193. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Liu, Z.; Zhu, Z.; Shi, J.; Jin, X.; Zhao, H.; Li, F.; Liu, S.; Wang, F. Blood Clearance Kinetics, Biodistribution, and Radiation Dosimetry of a Kit-Formulated Integrin αvβ3-Selective Radiotracer 99mTc-3PRGD2 in Non-Human Primates. Mol. Imaging Biol. 2011, 13, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Lucente, E.; Liu, H.; Liu, Y.; Hu, X.; Lacivita, E.; Leopoldo, M.; Cheng, Z. Novel 64Cu Labeled RGD2-BBN Heterotrimers for PET imaging of Prostate Cancer. Bioconjugate Chem. 2018, 29, 1595–1604. [Google Scholar] [CrossRef]
- Jiang, L.; Miao, Z.; Liu, H.; Ren, G.; Bao, A.; Cutler, C.S.; Shi, H.; Cheng, Z. 177Lu-labeled RGD-BBN heterodimeric peptide for targeting prostate carcinoma. Nucl. Med. Commun. 2013, 34, 909–914. [Google Scholar] [CrossRef]
- Liu, Z.; Radtke, M.A.; Wong, M.Q.; Lin, K.-S.; Yapp, D.T.; Perrin, D.M. Dual Mode Fluorescent 18F-PET Tracers: Efficient Modular Synthesis of Rhodamin-[cRGD]2-[18F]-Organotrifluoroborate, Rapid, and High Yielding One-Step 18F-Labeling at High Specific Activity, and Correlated in Vivo PET Imaging and ex Vivo Fluorescence. Bioconjugate Chem. 2014, 25, 1951–1962. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Moon, B.S.; Kim, J.S.; Jung, J.H.; Park, H.S.; Katzenellenbogen, J.A.; Kim, S.E. Synthesis and biological evaluation of RGD peptides with the 99mTc/188Re chelated iminodiacetate core: Highly enhanced uptake and excretion kinetics of theranostics against tumor angiogenesis. RSC Adv. 2013, 3, 782–792. [Google Scholar] [CrossRef]
- Hardee, M.E.; Zagzag, D. Mechanisms of Glioma-Associated Neovascularization. Am. J. Pathol. 2012, 181, 1126–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, I.H.; O’Donovan, D.G.; Brenchley, P.E.C.; Reid, H.; Roberts, I.S.D. Vascular endothelial growth factor expression correlates with tumour grade and vascularity in gliomas. Histopathology 2001, 39, 409–415. [Google Scholar] [CrossRef]
- Lamszus, K.; Ulbricht, U.; Matschke, J.; Brockmann, M.A.; Fillbrandt, R.; Westphal, M. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin. Cancer Res. 2003, 9, 1399–1405. [Google Scholar]
- Song, Y.S.; Park, H.S.; Lee, B.C.; Jung, J.H.; Lee, H.Y.; Kim, S.E. Imaging of integrin αvβ3 expression in lung cancers and brain tumors using single-photon emission computed tomography with a novel radiotracer 99mTc-IDA-D-[c(RGDfK)]2. Cancer Biother. Radiopharm. 2017, 32, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.S.; Kim, J.H.; Lee, B.C.; Jung, J.H.; Park, H.S.; Kim, S.E. Biodistribution and Internal Radiation Dosimetry of 99mTc-IDA-D-[c(RGDfK)]2 (BIK-505), a Novel SPECT Radiotracer for the Imaging of Integrin αvβ3 Expression. Cancer Biother. Radiopharm. 2018, 33, 396–402. [Google Scholar] [CrossRef]
- Yoo, J.S.; Lee, J.; Jung, J.H.; Moon, B.S.; Kim, S.; Lee, B.C.; Kim, S.E. SPECT/CT Imaging of High-Risk Atherosclerotic Plaques using Integrin-Binding RGD Dimer Peptides. Sci. Rep. 2015, 5, 11752. [Google Scholar]
- Mancuso, M.R.; Davis, R.; Norberg, S.M.; O’Brien, S.; Sennino, B.; Nakahara, T.; Yao, V.J.; Inai, T.; Brooks, P.; Freimark, B.; et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J. Clin. Investig. 2006, 116, 2610–2621. [Google Scholar] [CrossRef] [Green Version]
- Inai, T.; Mancuso, M.; Hashizume, H.; Baffert, F.; Haskell, A.; Baluk, P.; Hu-Lowe, D.D.; Shalinsky, D.R.; Thurston, G.; Yancopoulos, G.D.; et al. Inhibition of Vascular Endothelial Growth Factor (VEGF) Signaling in Cancer Causes Loss of Endothelial Fenestrations, Regression of Tumor Vessels, and Appearance of Basement Membrane Ghosts. Am. J. Pathol. 2004, 165, 35–52. [Google Scholar] [CrossRef] [Green Version]
- Henno, S.; Lambotte, J.C.; Glez, D.; Guigand, M.; Lancien, G.; Cathelineau, G. Characterisation and quantification of angiogenesis in β-tricalcium phosphate implants by immunohistochemistry and transmission electron microscopy. Biomaterials 2003, 24, 3173–3181. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, J.; Jia, B.; Yu, Z.; Liu, Y.; Zhao, H.; Li, F.; Tian, J.; Chen, X.; Liu, S.; et al. Two 90Y-Labeled Multimeric RGD Peptides RGD4 and 3PRGD2 for Integrin Targeted Radionuclide Therapy. Mol. Pharm. 2011, 8, 591–599. [Google Scholar] [CrossRef]
- Lucie, S.; Elisabeth, G.; Stéphanie, F.; Guy, S.; Amandine, H.; Corinne, A.R.; Didier, B.; Catherine, S.; Alexei, G.; Pascal, D.; et al. Clustering and Internalization of Integrin αvβ3 With a Tetrameric RGD-synthetic Peptide. Mol. Ther. 2009, 17, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Bourton, E.C.; Plowman, P.N.; Smith, D.; Arlett, C.F.; Parris, C.N. Prolonged expression of the γ-H2AX DNA repair biomarker correlates with excess acute and chronic toxicity from radiotherapy treatment. Int. J. Cancer 2011, 129, 2928–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, M.; Frielink, C.; Dijkgraaf, I.; Oyen, W.; Edwards, D.S.; Liu, S.; Rajopadhye, M.; Massuger, L.; Corstens, F.; Boerman, O. Improved Tumor Targeting of Radiolabeled RGD Peptides Using Rapid Dose Fractionation. Cancer Biother. Radiopharm. 2004, 19, 399–404. [Google Scholar] [PubMed] [Green Version]
- Jin, Z.-H.; Furukawa, T.; Ohya, T.; Degardin, M.; Sugyo, A.; Tsuji, A.B.; Fujibayashi, Y.; Zhang, M.-R.; Higashi, T.; Boturyn, D.; et al. 67Cu-Radiolabeling of a multimeric RGD peptide for αvβ3 integrin-targeted radionuclide therapy: Stability, therapeutic efficacy, and safety studies in mice. Nucl. Med. Commun. 2017, 38, 347–355. [Google Scholar] [CrossRef]
- Jun, H.T.; Sun, J.; Rex, K.; Radinsky, R.; Kendall, R.; Coxon, A.; Burgess, T.L. AMG 102, A Fully Human Anti-Hepatoxyte Growth Factor/Scatter Factor Neutralizing Antibody, Enhances the Efficacy of Temozolomide or Docetaxel in U-87 MG Cells and Xenografts. Clin. Cancer Res. 2007, 13, 6735–6742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johns, T.G.; Luwor, R.B.; Murone, C.; Walker, F.; Weinstock, J.; Vitali, A.A.; Perera, R.M.; Jungbluth, A.A.; Stockert, E.; Old, L.J.; et al. Antitumor efficacy of cytotoxic drugs and the monoclonal antibody 806 is enhanced by the EGF receptor inhibitor AG1478. Proc. Natl. Acad. Sci. USA 2003, 100, 15871–15876. [Google Scholar] [CrossRef] [Green Version]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.L.; Porto-Carreiro, I.; Pereira, C.M.; Balca-Silva, J.; Kahn, S.A.; Dos Santos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 12, 418. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.H.; Choi, J.Y.; Jung, J.H.; Song, I.H.; Park, H.S.; Denora, N.; Leonetti, F.; Kim, S.E.; Lee, B.C. Effect of Peptide Receptor Radionuclide Therapy in Combination with Temozolomide against Tumor Angiogenesis in a Glioblastoma Model. Cancers 2021, 13, 5029. https://doi.org/10.3390/cancers13195029
Lee SH, Choi JY, Jung JH, Song IH, Park HS, Denora N, Leonetti F, Kim SE, Lee BC. Effect of Peptide Receptor Radionuclide Therapy in Combination with Temozolomide against Tumor Angiogenesis in a Glioblastoma Model. Cancers. 2021; 13(19):5029. https://doi.org/10.3390/cancers13195029
Chicago/Turabian StyleLee, Sang Hee, Ji Young Choi, Jae Ho Jung, In Ho Song, Hyun Soo Park, Nunzio Denora, Francesco Leonetti, Sang Eun Kim, and Byung Chul Lee. 2021. "Effect of Peptide Receptor Radionuclide Therapy in Combination with Temozolomide against Tumor Angiogenesis in a Glioblastoma Model" Cancers 13, no. 19: 5029. https://doi.org/10.3390/cancers13195029
APA StyleLee, S. H., Choi, J. Y., Jung, J. H., Song, I. H., Park, H. S., Denora, N., Leonetti, F., Kim, S. E., & Lee, B. C. (2021). Effect of Peptide Receptor Radionuclide Therapy in Combination with Temozolomide against Tumor Angiogenesis in a Glioblastoma Model. Cancers, 13(19), 5029. https://doi.org/10.3390/cancers13195029